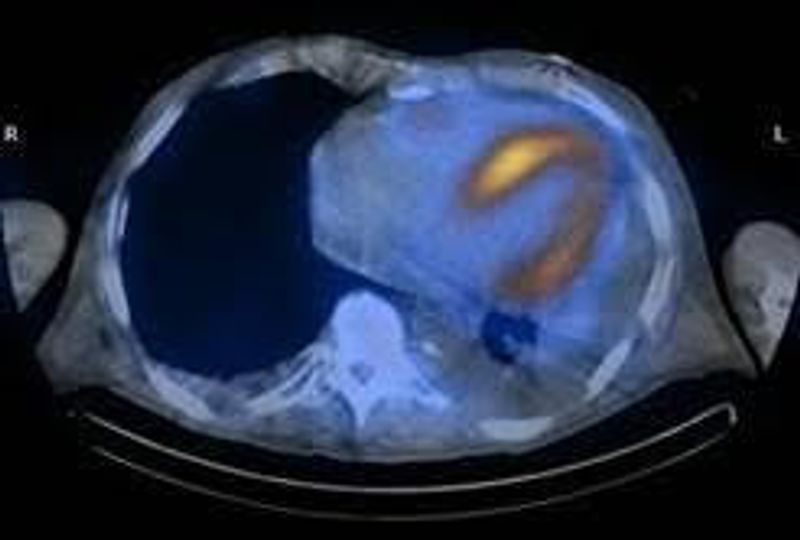
“For in vivo shipment, the objective is that you can administer CRISPR as a medication to the client,” said Laura Sepp-Lorenzino, primary scientific officer of Intellia Therapeutics.ATTR is characterized by a misfolded variation of the transthyretin (TTR) protein that develops up in the heart, worried system, and kidneys. These treatments often fall short of treating the disease; while symptoms improve, numerous clients die within 10 years of diagnosis.ATTR can lead to the build-up of TTR protein in various organs, specifically the heart (revealed in yellow). Future plans are to continue increasing the dose, which Gilmore anticipates will lead to better outcomes for more patients.While initial trial results focus on patients with nerve damage, another class of patients has primarily cardiac symptoms. Gilmore believes that these clients may benefit even more from this treatment due to the fact that amyloid accumulation in the heart is especially responsive to TTR levels. There are strategies to check the drug in these clients in the next phase of trials.If the drug continues to prosper in medical trials, Sepp-Lorenzino expects that treatment for ATTR clients might one day be as simple as a two-hour infusion of the lipid nanoparticles at an outpatient.
Preliminary arise from a continuous trial by Intellia Therapeutics reveal that a CRISPR-Cas9-based drug can be delivered into the body to reduce and target the liver expression of the gene that causes transthyretin amyloidosis (ATTR).1 This is the first scientific trial showing effective in vivo gene editing; the outcomes recommend that it may be possible to safely modify the genomes of cells in the body. “For in vivo shipment, the goal is that you can administer CRISPR as a medication to the client,” stated Laura Sepp-Lorenzino, primary clinical officer of Intellia Therapeutics.ATTR is identified by a misfolded version of the transthyretin (TTR) protein that constructs up in the heart, nerve system, and kidneys. Patients usually experience pain, weakness, and the failure to manage fundamental body functions. “Its a truly awful condition,” said Julian Gilmore, a physician-scientist at the National Amyloidosis Centre at University College London, who led the trial.Past research studies revealed a possibly straightforward path to treatment: lower the amount of TTR in circulation to lower disease signs. Lower TTR levels slow down protein accumulation. Some formerly authorized drugs achieve this with little interfering RNAs or antisense oligonucleotides that help deteriorate or sequester the RNA encoding TTR.2,3 But these treatments require a pre-treatment followed by weekly or month-to-month administration of the drug, which causes undesirable adverse effects. These treatments often fall brief of treating the disease; while symptoms improve, lots of clients pass away within 10 years of diagnosis.ATTR can cause the accumulation of TTR protein in various organs, particularly the heart (shown in yellow). Julian GillmoreThe potential to deal with ATTR by simply decreasing TTR– and the restricted effectiveness of prior efforts to do so– made the disease a perfect circumstance for CRISPR-Cas9, Gilmore said. The molecular scissors might reduce TTR levels by interrupting the gene that encodes it. This snip is a more effective method to dampen expression, and a single infusion of CRISPR edits the cells to decrease TTR in eternity, which could likewise fix prior damage. “The goal for Intellia is not only to halt progression of the illness, but potentially to be able to go back and get function,” said Sepp-Lorenzino. Utilizing CRISPR to edit cells straight in the body– instead of eliminating and modifying cells in the laboratory and returning them to the client– is no small job. Here, ATTR provided a special advantage. TTR is produced nearly exclusively in the liver, an organ that can be particularly targeted for drug shipment. Wrapping a drug in a bubble of fats called a lipid nanoparticle triggers it to head straight to the liver, enabling efficient shipment and decreasing off-target effects.Intellias lipid nanoparticles consist of a guide RNA targeting TTR and mRNA encoding the Cas9 endonuclease that makes the genomic cuts. In their ongoing Phase I trial, they administered the drug (NTLA-2001) to 6 clients with nerve damage resulting from ATTR. Half of the individuals got a lower dose and half got a higher dosage. Twenty-eight days after treatment, there were no reports of extreme negative results.”I dont believe theres any question based upon the outcomes that the editing not only works, however works extremely well,” stated Kiran Musunuru, a cardiologist at the University of Pennsylvania and co-founder of the CRISPR biotechnology business Verve Therapeutics, who was not associated with the study.TTR levels were cut in half in the lower-dose group, and decreased by 80-96 percent in the higher-dose group. The goal is to minimize TTR by 90-95 percent, Gilmore stated. For contrast, existing therapies lower its existence by roughly 80 percent. He was amazed to see that some patients in the trial reached these preferred levels currently. “It opens up the genuine possibility that these clients are in fact going to improve,” Gilmore said. Future plans are to continue increasing the dosage, which Gilmore anticipates will lead to much better results for more patients.While preliminary trial results focus on patients with nerve damage, another class of clients has primarily cardiac symptoms. Due to the fact that amyloid accumulation in the heart is especially responsive to TTR levels, Gilmore believes that these patients may benefit even more from this treatment. There are strategies to evaluate the drug in these patients in the next phase of trials.If the drug continues to succeed in medical trials, Sepp-Lorenzino anticipates that treatment for ATTR clients may one day be as simple as a two-hour infusion of the lipid nanoparticles at an outpatient center.”Its going to move from a model where were taking pills every day or injections every few weeks for long periods of time,” Musunuru said. “Gene modifying treatments are going to transform that model into one where you have one-and-done therapies.”Demonstrating the ability to provide genome modifying equipment to the liver possibly unlocks to modifying any gene in the liver and to treating more conditions. Intellia is currently dealing with another in vivo CRISPR drug for hereditary angioedema that knocks out a different gene in liver cells.It will take more work to target other cell types. Scientists may craft lipid nanoparticles with particular molecules on their surface to connect with a target cell type. An in vivo scientific trial performed by Editas, a biotechnology business creating CRISPR-based therapeutics, to deal with a retinal degeneration condition with virus-delivered CRISPR-Cas9 also recently showed medical advantage, although retinal editing doesnt have as clear of a readout as TTR levels.4″If we think about CRISPR-Cas9 as revolutionizing medicine, then what we require is to be able to deliver the drug to whichever cells in the body we want,” Gilmore stated. “I think thats the real obstacle for the larger application of this innovation.”ReferencesJ.D. Gillmore et al., “CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis,” N Engl J Med, 385( 6 ):493 -502, 2021. D. Adams et al., “Patisiran, an RNAi healing, for genetic transthyretin amyloidosis,” N Engl J Med, 379( 1 ):11 -21, 2021. M.D. Benson et al., “Inotersen treatment for clients with genetic transthyretin amyloidosis,” N Engl J Med, 379( 1 ):22 -31, 2021. J. Mullen et al., “BRILLIANCE: A phase 1/2 single ascending dosage research study of EDIT-101, an in vivo CRISPR gene editing treatment in CEP290-related retinal degeneration,” Presentation, September 29, 2021.