Group members there were carrying out such experiments when they discovered that a particular protein, when introduced into the cell, would end up being displaced from the cell growth area. They describe these outcomes in a paper called “Cell pattern by secretion-induced plasma membrane flows” in a recent issue of Science Advances.
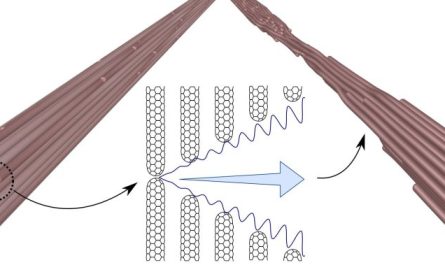
Image portraying the impact of membrane circulation on the distribution of cell membrane– associated proteins. When cells move or grow, they need to include new membrane to those development areas, states Vavylonis.
This multidisciplinary partnership combined modeling and experiments to explain a previously-unknown biological procedure. The groups found and characterized a brand-new system that an easy yeast cell utilizes to get its shape. They describe these lead to a paper called “Cell pattern by secretion-induced plasma membrane streams” in a recent issue of Science Advances.
Image illustrating the influence of membrane circulation on the distribution of cell membrane– associated proteins. Proteins (green) with low movement couple to the circulations and are diminished from the secretion zone. Credit: Lehigh University and University of Lausanne
When cells move or grow, they need to add brand-new membrane to those development areas, says Vavylonis. The process of membrane shipment is called exocytosis. Cells likewise need to deliver this membrane to a specific location in order to maintain a sense of direction-called “polarization”- or grow in a collaborated manner.
” We showed that these processes are combined: local excess of exocytosis triggers a few of the proteins connected to the membrane to move ( flow) away from the growth region,” says Vavylonis. “These proteins that move away mark the non-growing cell region, thus developing a self-reliant pattern, which triggers the tubular shape of these yeast cells.”
This is the very first time that this mechanism for cell patterning-the procedure by which cells get spatial nonuniformities on their surfaces-has been recognized.
The Vavylonis groups simulations, spearheaded by Postdoctoral Associate David Rutkowski, caused speculative tests which the Martin group then carried out. Vavylonis and Rutkowski evaluated the results of the experiments to confirm that the circulation of proteins they observed in their simulations matched the data gleaned from the experiments on live cells.
The team states that the work might be of particular interest to scientists investigating processes that associate with cell growth and membrane traffic such as neurobiologists and those studying cancer cell procedures.
” Our work shows that patterns in biological systems are usually not static,” says Rutkowski. “Patterns develop themselves through physical procedures involving constant circulation and turnover.”
” We were able to supply support for the design of patterning by membrane-flow,” stated Vavylonis. “In the end, the Martin group had the ability to use this knowledge to engineer cells whose shape can be controlled by light.”
Referral: “Cell patterning by secretion-induced plasma membrane streams” by Veneta Gerganova, Iker Lamas, David M. Rutkowski, Aleksandar Vještica, Daniela Gallo Castro, Vincent Vincenzetti, Dimitrios Vavylonis and Sophie G. Martin, 17 September 2021, Science Advances.DOI: 10.1126/ sciadv.abg6718.
Schizosaccharomyces pombe yeast cells dividing.
A multi-disciplinary group of researchers at Lehigh University and the University of Lausanne find and characterize a new mechanism by which the fission yeast cell obtains its tubular shape.
Working with light to activate processes within genetically customized fission yeast cells is amongst the research performed by the speculative biologists in the Martin Lab at the University of Lausanne, led by faculty member Sophie Martin. Employee there were performing such experiments when they observed that a specific protein, when introduced into the cell, would end up being displaced from the cell growth area. So, they connected to Dimitrios Vavylonis, who leads the Vavylonis Group in the Department of Physics at Lehigh University, to learn why.
” We proceeded to make a computational simulation that paired cell membrane growth to protein motion as well as design a few other hypotheses that we considered after discussions with them,” says Vavylonis, a theoretical physicist.