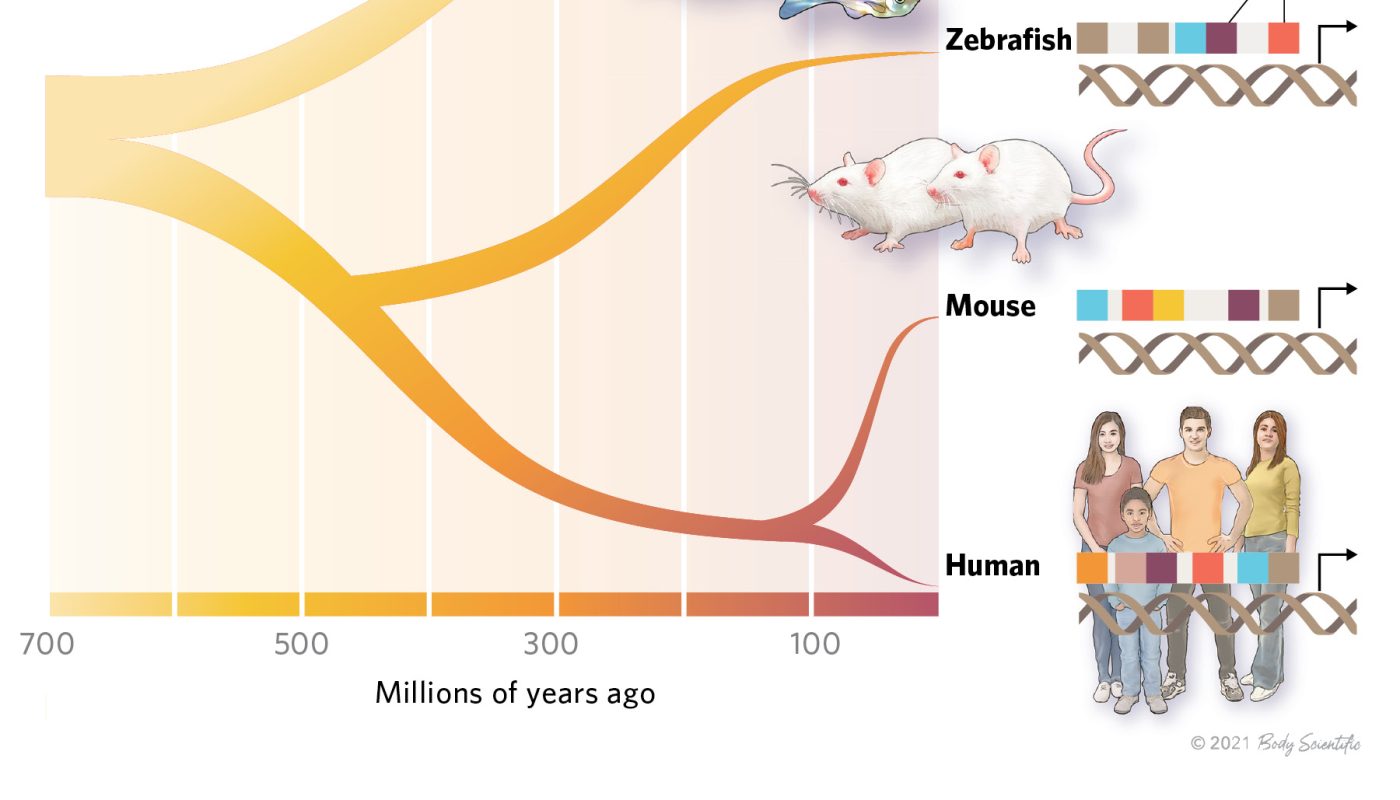
About 700 million years back, sponges branched off from all other animals on the tree of life. In spite of this evolutionary distance, sponges share a kind of gene policy with a lot more intricate species. The systems are so similar, in truth, that a hereditary element called an enhancer from the sea sponge Amphimedon queenslandica can drive transcription in particular cell key ins mice and zebrafish, despite the reality that the genomes of these animals do not normally consist of a comparable series, according to a study released late in 2015 in Science.The result was completely surprising, states Emily Wong, a computational genomics scientist at Victor Chang Cardiac Institute in Australia and a coauthor of the study. “We didnt believe it would work.”The outcomes work as an extreme example of what scientists are now acknowledging as trends amongst enhancers– that activity can be conserved over long evolutionary timescales which such saved activity doesnt require DNA sequences to match completely. “Just the large distance between the species … makes that [gene regulatory activity] actually interesting and cool,” says Tony Capra, an evolutionary geneticist at the University of California, San Francisco, who was not involved in the research.It is really made complex. And that, obviously, makes it intriguing and fun.– Paul Flicek, European Molecular Biology LaboratoryWhile there are some examples of “ultraconserved” enhancer aspects that are identical in rodents and humans, increased genome sequencing has actually exposed that enhancers typically develop rapidly, collecting substantial sequence modifications over fairly brief periods. Enhancers have maintained molecular relationships with the proteins that regulate transcription, many of which have actually been maintained over hundreds of millions of years. The guidelines underlying how enhancer sequences interact with these proteins to regulate transcription stay dirty, and scientists are still arranging out the information of enhancer biology and trying to comprehend how function is conserved even as the DNA code mutates. “It is actually made complex,” says Paul Flicek, a computational biologist at the European Molecular Biology Laboratorys European Bioinformatics Institute in the UK. “And that, obviously, makes it interesting and enjoyable.”Enhancer grammarEnhancers contain transcription aspect (TF) binding websites and regulate transcription at a distance– sometimes a very country mile. In mice and humans, for instance, an enhancer component for the Shh gene, associated with patterning throughout embryonic advancement, is situated about 1 million base sets away, and anomalies in this enhancer element can trigger organisms to develop additional fingers or toes. Beyond those universal qualities, enhancers differ dramatically. They can be discovered upstream or downstream of genes, or perhaps within them. Enhancers likewise vary in length, varying from about 10 to 1,000 base pairs, and contain various numbers of TF binding websites. TFs glom on to the DNA and hire parts of the transcriptional machinery to promoters, stretches of the genome where gene transcription is started. How exactly TFs put together at enhancers to influence gene guideline remains a little a black box, however.One concept, called the enhanceosome design, presumes that a specific variety of TFs and other proteins should be present in a defined orientation to affect gene expression. These guidelines are also understood as enhancer grammar. A traditional example of this design is the interferon-β enhancer, where eight proteins, including 3 TFs, must exist on the DNA to direct the transcriptional machinery to the interferon-β genes promoter. In an alternative “signboard” model, TF binding to enhancer sequences doesnt depend on grammar: the number, order, and spacing can vary and still impact gene expression, in some cases in various ways. “Its truly that these are two ends of a spectrum,” says Emma Farley, a molecular biologist at the University of California, San Diego. A third concept, called the TF-collective design, includes another layer of complexity by proposing that transcription elements bound to DNA hire additional TFs through protein-protein interactions; these extra TFs could influence transcription without binding defined DNA series. (See illustration listed below.)All these models for enhancer function, varying from rigid enhancer grammar to flexible grammar to no grammar, could be proper, depending on biological context. “We require to understand why different kinds of enhancers fall at different places on that spectrum,” Farley says. “That may assist us understand the various nuances of enhancer grammar much better.”Because theres no means of identifying enhancers by series alone, researchers determine regions of regulative activity based on indirect readouts. For instance, active enhancers are generally related to open chromatin– stretches of DNA not firmly wound around nucleosomes. Another technique examines DNA associated with certain variations of histones, the proteins that comprise nucleosomes. Particular patterns of histone modifications decorate enhancer areas. For both methods, biochemical recognition and sequencing of targeted areas can yield putative enhancers. The outcomes generated by such methods dont constantly agree, notes Capra, but “I do not see that as necessarily a problem. I think it simply shows that we are studying an extremely complex biochemical process that has many inputs and outputs [and] several signatures that it leaves along the genome over time.” That said, he adds in an email, “the argument is a problem when researchers do not account for it.”Enhancer FunctionEnhancers mediate gene expression by recruiting transcription aspects (TFs) that consequently hire extra equipment to initiate transcription. Enhancers typically act throughout country miles in the genome– approximately about 1 million base pairs, as holds true for the developmental patterning gene Shh– and their location relative to the genes they manage varies: they can be upstream or downstream, and they can reside beyond coding locations entirely or within introns of other genes. © BODY SCIENTIFIC INTERNATIONALSee complete infographic: WEBConservation of enhancer activityAs shown by the sponge enhancer, conserved activity does not depend on identical DNA sequences. However there are cases where evolution has actually protected the precise series of enhancer aspects in distantly associated types. A striking example of how enhancers can be conserved at the sequence level are “ultraconserved” elements, initially described in 2004 by collaborators at the University of California, Santa Cruz, and the University of Queensland in Australia. Their study revealed 481 segments longer than 200 base pairs that were perfect matches across human, rat, and mouse genomes; later studies on 245 of these elements located in noncoding stretches of the human genome found that about half had enhancer activity and might drive gene expression throughout mouse development.The discovery of ultraconserved components was astounding considering that about 80 million years separate people and rodents from our last common ancestor. The scientists calculated that, based on even a sluggish anomaly rate, the probability that a person such genetic sequence would by opportunity exist in the around 3 billion bases of the human genome would be less than 1 in 1022. Scientists assumed that ultraconserved sequences should be essential. They were wrong: mice stayed viable and fertile even when some of these enhancers were gotten rid of.”People were actually sort of stunned by it,” says Diane Dickel, a genomicist at Lawrence Berkeley National Laboratory who did not participate in that work. She was associated with later studies that discovered that eliminating ultraconserved enhancers did have a result– it was simply subtle. More just recently, she and her associates mutated 23 ultraconserved enhancers and discovered that, for the a lot of part, they still had activity throughout development. The findings recommend that even these extremely conserved enhancers can still endure series changes, which begs the question of why more variation hasnt crept in over countless years. “We still dont have a totally clear understanding about why these websites are so perfectly conserved,” Dickel says.But ultraconserved elements are an exception in enhancer advancement. In a landmark study in 2015 that tracked promoters and enhancers throughout 20 mammalian species using histone marks, the researchers found that promoters developed gradually while enhancers progressed rapidly. In spite of a lack of preservation in general sequence, enhancers from distantly related species do have something in typical: TF binding site series. Transcription factors are deeply saved throughout the tree of life. A comparison of TFs in fruit flies, human beings, and mice exposed that the different variations of the proteins had similar binding properties and acknowledged the exact same DNA sequences. This preservation could be partially why the A. queenslandica enhancer operated in mice and zebrafish despite hundreds of millions of years of evolutionary range. While the enhancer sequences didnt align general in that research study, common brief TF binding concepts appeared in the zebrafish, sponge, and mouse enhancers, albeit in different arrangements.Just the large distance in between the types … makes that [gene regulatory activity] actually amazing and cool.– Tony Capra, University of California, San FranciscoSequence similarity or not, “the reality that these enhancers work really means that animals over these large ranges share what one may call a cell type,” specified by a typical set of TFs, states Alexander Stark, a genomicist at the Research Institute of Molecular Pathology in Austria who was not involved in the sponge study. This shared cell type was apparently preserved across evolutionary time in distantly related species, he continues, and TFs acknowledge saved binding concepts, despite their rearrangements in the different animal genomes. “The relative position and orientation of these brief concepts with respect to each other frequently does not [seem to] matter,” Stark says. There are likewise some cases where it does. Experiments using crafted enhancers from the sea squirt Ciona intestinalis, for instance, discovered that modifications in the spacing or arrangement of binding concepts could compensate for weak binding websites to drive gene expression.Evolution is acting on what genes are turned on and when, Capra says. “Evolution doesnt really care what specific sequences along the genome are driving that activity. Its putting selection on the output of gene expression.”Another reason enhancer function is frequently conserved in spite of series changes is redundancy. These hereditary elements can have several copies of TF binding sites, so modifications to one site might not adversely affect transcription– particularly with the billboard design. There may likewise be more than one enhancer acting upon a gene. These integrated backups might permit mutations to collect without apparent functional effects. These qualities do make enhancers tough to study, states Flicek.Analyses of enhancers usually involve knocking out individual TF binding sites. Imagine a situation with 20 people pressing a cars and truck up a hill, Flicek states. Eliminating one person might lower performance a little, however the car might still make it up the hill. You may conclude that the missing individual wasnt necessary. You might conclude that none of them were vital if you went through this process with all 20 individuals. “But thats not whats actually occurred,” Flicek states. “Whats actually occurred existss redundancy that we do not see, and we cant comprehend.”Enhancer EvolutionEnhancers are stretches of DNA that control where and when a gene is expressed. While the sequences of enhancers can vary among species, their function is highly conserved across numerous countless years of evolution. For example, a current study discovered that an enhancer from the sponge Amphimedon queenslandica can drive transcription in specific cell types in mice and zebrafish. While enhancers in the more intricate organisms didnt match the series of the sponge enhancer, the areas included different plans of shared transcription aspect binding concepts. The very same was also true in the human region that the majority of carefully matched the sponge enhancer. © BODY SCIENTIFIC INTERNATIONALSee full infographic: WEBEnhancers with time While many enhancers do progress rapidly, there are still constraints on how much they alter. The A. queenslandica enhancer that drove activity in mice and zebrafish, for instance, is positioned within a bystander gene different from the one whose expression it modified. Such a plan might restrict just how much the enhancer can alter over evolutionary timescales without disrupting the function of that onlooker gene.Similarly, enhancers involved in numerous biological processes tend to have more-conserved activity. In a research study examining enhancers active in liver tissue throughout 10 mammalian species, researchers discovered that, in general, the more traits or cellular contexts in which an enhancer contributed, the more likely it was to have activity in all 10 species.Scientists continue to investigate what other selective pressures have driven enhancer evolution over numerous countless years and to break the secrets of the regulative code. “The field is just in an actually interesting location right now, with lots of completing designs [and] great deals of uncertainty,” Capra says. “And just truly cool results.”Editors note: Before work on this post was total, Jack J. Lee started an interactions fellowship at the National Cancer Institutes Division of Cancer Prevention.
— Paul Flicek, European Molecular Biology LaboratoryWhile there are some examples of “ultraconserved” enhancer aspects that are identical in rodents and human beings, increased genome sequencing has revealed that enhancers often develop quickly, accumulating considerable sequence changes over reasonably brief durations. These qualities do make enhancers challenging to study, states Flicek.Analyses of enhancers usually include knocking out private TF binding sites. While enhancers in the more intricate organisms didnt match the series of the sponge enhancer, the regions included different arrangements of shared transcription aspect binding concepts. Such an arrangement might limit how much the enhancer can change over evolutionary timescales without interfering with the function of that onlooker gene.Similarly, enhancers involved in lots of biological procedures tend to have more-conserved activity. In a research study examining enhancers active in liver tissue across 10 mammalian species, scientists found that, in basic, the more characteristics or cellular contexts in which an enhancer played a role, the more likely it was to have activity in all 10 species.Scientists continue to investigate what other selective pressures have driven enhancer development over hundreds of millions of years and to split the secrets of the regulatory code.