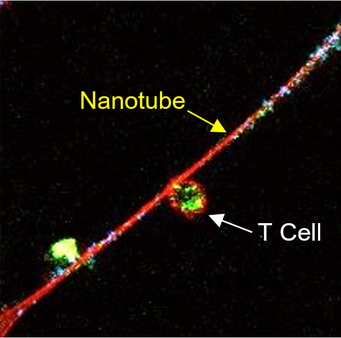
A T cell (upper left) connected to a cancer cell (lower right) by a nanotube@Tanmoy SahaCancer cells send nanotubes to draw mitochondria from immune cells, finds a November 18 study in Nature Nanotechnology. The pilfered organelles allow the cancer cells to replenish their power while deteriorating T cells– a finding that might lead to brand-new opportunities for assaulting tumors. “Its surprising that the transfer of mitochondria took place in between various cell types, intriguingly between immune cells and cancer cells,” writes cancer biologist Ming Tan of China Medical University in Taiwan, who was not included in this study, in an e-mail to The Scientist. While researchers have actually observed mitochondrial transfer between cells before, a lot of cases happened between two cells of the exact same type. “Moreover, the mitochondrial transfer appears to have a considerable effect on tumor cells getting away from immune monitoring,” Tan adds. “This is interesting due to the fact that [of] its prospective restorative implications.”See “Nanotubes Link Immune Cells”Shiladitya Sengupta and associates at Brigham and Womens Hospital and MIT, including co-corresponding author Hae Lin Jang, came across the habits of these nanotubes as they were taking a more comprehensive view of cancer characteristics. “What we are attempting to do is construct a design system to study how cancer cells and immune cells communicate utilizing tools from nanotechnology, entering into resolutions not possible with light microscopy. […] And we saw these tubes.” Additional examination with field-emission scanning electron microscopy exposed a variety of these tiny connectors, from 3 to 100µm in length and 50nm to 2µm in width, in between cultured mouse and human breast cancer cells and mouse T cells. Mitochondria (green) traveling from a T cell toward a cancer cell through a nanotube (red)To determine what cargo passes through televisions, Sengupta and associates labeled mitochondria with MitoTracker Green and amine-containing particles in the cytoplasm with CellTrace Far Red. While some cytoplasmic proteins slowly moved in between cells, mitochondria took a trip much quicker. “Mitochondrial trafficking was likewise more unidirectional: About 90 percent of traffic is from the immune cell to the cancer cell,” says Sengupta. “Clearly, the truth that mitochondrial trafficking is faster means that it is an active procedure that is happening.” The group likewise found that cancer cells grown in culture with T cells taken in about two times as much oxygen as the same cells grown on their own, suggesting that mitochondria pilfered from the immune cells offered the cancer cells a metabolic boost. The cocultured T cells respiration was about half that of T cells grown alone. “The idea that [cancer cells] can coopt regional immune cells and obtain mitochondria and deplete them … is very amazing,” says oncologist Emil Lou of the University of Minnesota Medical School, who was not associated with the research study. “This research study opens the possibility that the cancer cells are … not just not permitting immune cells to have an anticancer result but loaning from them and flourishing a lot more because of the immune cells, through the nanotubes.”Tan writes that he questions whether cancer cells may be taking other types of freight from immune cells released to combat the quickly dividing invaders. “It would be intriguing to know what other organelles or other cellular products next to mitochondria can be transferred through nanotubes, and what their effect on cancer cells and immune cells [might be]” See “Cancerous Conduits”Currently, no particular inhibitors are available to obstruct the formation of nanotubes. So, to test how hindering mitochondrial transfer affects cancer cells, the scientists hindered Ras/Rho GTPase signaling rather, which provides the “fuel” for the formation of nanotubes. The inhibitor stopped nanotubes from forming in a culture of cancer and immune cells, though it likely had other impacts on both the cancer and immune cells as well, as Ras/Rho GTPase signaling is involved in managing of gene expression, cell cycle progression, and cell migration. When the scientists injected the inhibitor alone into mouse models of human breast cancer, however, tumor development was not decreased. The researchers did observe a reduction in growth when the Ras/Rho GTPase inhibitor was combined with a PD1 inhibitor, an immunotherapy drug that obstructs cancer cells from “silencing” immune cells. Sengupta points out that not all patients react to such drugs now. “Maybe in those clients, the tumor is drawing the mitochondria out and shutting [the immune cells] down … If I can avoid that, I d get more immune cells in the tumor, and thats the goal.”Sengupta composes in an e-mail that he and his coworkers at Brigham and Womens are establishing more-specific inhibitors of nanotube formation, and have filed a patent. Sengupta also cofounded Akamara Therapeutics and Invictus Oncology, both of which are actively pursuing immune-based cancer therapies.Lou states the hypothesis that the Ras/Rho GTPase inhibitors tumor-shrinking effect in mice comes from avoiding nanotube formation needs more robust support. “That the exact result that happened in the mouse model … occurred due to the fact that of nanotubes is challenging to state based upon the information supplied,” he states, adding that although he concerns the research study as “really in-depth,” it lacked engaging, in vivo evidence of nanotubes forming in growths.
“Its unexpected that the transfer of mitochondria took place in between various cell types, intriguingly between immune cells and cancer cells,” composes cancer biologist Ming Tan of China Medical University in Taiwan, who was not involved in this study, in an e-mail to The Scientist. “What we are attempting to do is construct a design system to study how cancer cells and immune cells communicate using tools from nanotechnology, going into resolutions not possible with light microscopy. The team also discovered that cancer cells grown in culture with T cells consumed about two times as much oxygen as the exact same cells grown by themselves, indicating that mitochondria pilfered from the immune cells gave the cancer cells a metabolic boost. “This research study opens up the possibility that the cancer cells are … not only not enabling immune cells to have an anticancer impact however loaning from them and prospering even more since of the immune cells, through the nanotubes.”Tan writes that he wonders whether cancer cells might be stealing other forms of freight from immune cells deployed to combat the quickly dividing intruders.