Other conditions that feature persistent brain inflammation afflict 10s of millions more and consist of Alzheimers and Parkinsons illness. There is likewise proof that neuroinflammation develops naturally with aging and is a major factor in age-related cognitive decline, and more just recently inflammatory T-cell responses in the brain have actually been linked to neurological symptoms associated with SARS-CoV-2 infection.
The researchers have shown in recent work that ILC3s residing in the gut act as guards and immune regulators, suppressing swelling– including inflammatory T-cell activity– and warding off cancer. In the brand-new study, they examined the functions of ILC3s in the brain, and found, contrary to their expectation, that ILC3s are not normally present in the brain under healthy conditions but can penetrate the brain from the blood stream during inflammation. Support for human sample acquisition through the JRI IBD Live Cell Bank is supplied by the JRI, Jill Roberts Center for IBD, Cure for IBD, the Rosanne H. Silbermann Foundation and Weill Cornell Medicine Division of Pediatric Gastroenterology and Nutrition.
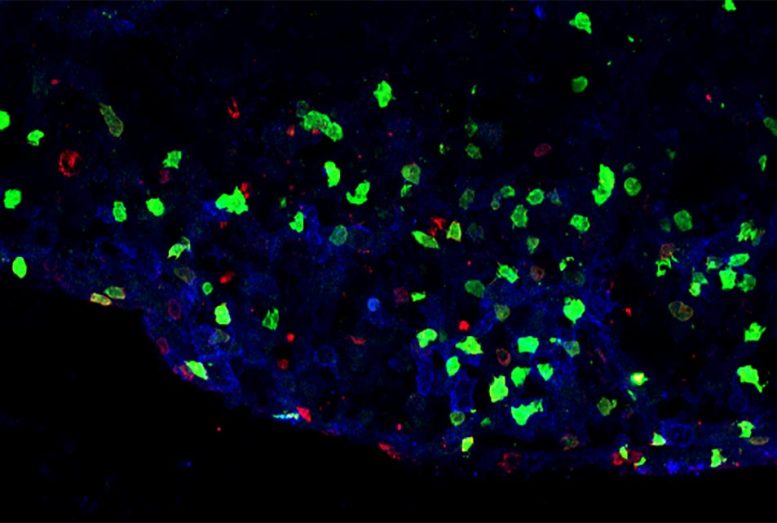
Inflammatory sore in the spine of a mouse design of numerous sclerosis showing the presence of ILC3 (green) or T cells (red). Credit: Image courtesy of Dr. Christopher N. Parkhurst
A group of immune cells that typically safeguard against inflammation in the gastrointestinal system might have the opposite effect in numerous sclerosis (MS) and other brain inflammation-related conditions, according to a new study by Weill Cornell Medicine and NewYork-Presbyterian researchers. The outcomes recommend that countering the activity of these cells might be a new restorative approach for such conditions.
The researchers, who reported their findings on December 1, 2021, in the journal Nature, were studying a set of immune cells called group 3 inherent lymphoid cells (ILC3s), which assist the immune system endure advantageous microbes and reduce inflammation in the intestines and other organs throughout the body. They found an unique subset of these ILC3s that distribute in the bloodstream and can infiltrate the brain– and, to their surprise, do not satiate inflammation but rather spark it.
The researchers called this subset inflammatory ILC3s, and found them in the central worried system of mice with a condition modeling MS. Instead of constraining the immune action, this subset of ILC3s stimulated another group of immune cells called T cells to attack myelinated nerve fibers, causing MS-like illness symptoms. The scientists identified comparable inflammatory ILC3s in the peripheral blood and cerebrospinal fluid of MS patients.
” This work has the potential to inform our understanding of, and potential treatments for, a broad variety of conditions involving T-cell seepage of the brain,” stated senior author Dr. Gregory Sonnenberg, associate professor of microbiology and immunology in medication in the Division of Gastroenterology and Hepatology and a member of the Jill Roberts Institute for Research in Inflammatory Bowel Disease at Weill Cornell Medicine.
MS affects more than two million individuals worldwide. Other conditions that feature persistent brain inflammation afflict tens of millions more and include Alzheimers and Parkinsons diseases. There is likewise proof that neuroinflammation develops naturally with aging and is a major consider age-related cognitive decline, and more recently inflammatory T-cell reactions in the brain have actually been connected to neurological symptoms connected with SARS-CoV-2 infection.
The researchers have revealed in current work that ILC3s residing in the gut function as guards and immune regulators, reducing swelling– consisting of inflammatory T-cell activity– and warding off cancer. In the new study, they analyzed the roles of ILC3s in the brain, and discovered, contrary to their expectation, that ILC3s are not generally present in the brain under healthy conditions but can penetrate the brain from the bloodstream during swelling. When they do penetrate the main anxious system, they have pro-inflammatory rather than anti-inflammatory results.
The researchers showed with a mouse model of MS that these inflammatory ILC3s in the brain function as antigen-presenting cells: They display bits of myelin protein, the main active ingredient in the insulating layer around nerve fibers, to T cells– prompting them to attack myelin, causing the nerve damage that triggers illness indications. They discovered the inflammatory ILC3s in close association with T cells in areas of active inflammation and nerve damage in the mouse brains.
” The seepage of these inflammatory ILC3s to the brains and spinal cords of mice accompanies the onset and peak of illness,” stated first author John Benji Grigg, a Weill Cornell Graduate School of Medical Sciences doctoral candidate in the Sonnenberg lab. “Further, our speculative information in mice show these immune cells play a key role in driving the pathogenesis of neuro-inflammation.”
The scientists found that they could prevent MS-like disease in the animals by removing from the ILC3s a key molecule called MHCII, which usually is utilized in the antigen-presenting procedure– the removal basically obstructs the cells capability to trigger myelin-attacking T cells.
” Despite our absolute best disease-modifying treatments for MS, patients continue to progress, and because disease onset is early in life, they deal with the possibility of permanent physical and cognitive disability,” said co-author Dr. Tim Vartanian, teacher of neuroscience in the Feil Family Brain and Mind Institute at Weill Cornell Medicine, chief of the division of numerous sclerosis and neuro-immunology and a teacher of neurology in the Department of Neurology at Weill Cornell Medicine and NewYork-Presbyterian/Weill Cornell Medical Center. “Identification of inflammatory ILC3s with antigen discussion capabilities in the main nerve system of individuals with MS offers a new strategic target to prevent nervous system injury.”
The researchers discovered that ILC3s that reside in other tissues in the body can be configured, in effect, to counter the activity of brain-infiltrating T cells, avoiding the MS-like condition illness in mice.
This work was completed in close collaboration with Dr. Ari Waisman, director of the Institute for Molecular Medicine at the University Medical Center of Johannes Gutenberg University Mainz, where the researchers built on previous research showing that there are gut-resident ILC3s that display antigens to T cells in a slightly various method to promote T-cell inactivity, or “tolerance.” The scientists showed that by experimentally exposing these tolerance-inducing digestive ILC3s to myelin, they might obstruct neuroinflammatory T-cell activity and the development of MS-like illness in the mice.
The work therefore indicates the possibility that MS and potentially many other inflammatory conditions could someday be dealt with either by directly inhibiting the activity of inflammatory ILC3s that penetrate the brain, or by targeting self-antigens to the intestinal tract ILC3s that promote tolerance in other tissues, Dr. Sonnenberg said.
Recommendation: “Antigen-presenting inherent lymphoid cells orchestrate neuroinflammation” by John B. Grigg, Arthi Shanmugavadivu, Tommy Regen, Christopher N. Parkhurst, Anees Ahmed, Ann M. Joseph, Michael Mazzucco, Konrad Gronke, Andreas Diefenbach, Gerard Eberl, Timothy Vartanian, Ari Waisman and Gregory F. Sonnenberg, 1 December 2021, Nature.DOI: 10.1038/ s41586-021-04136-4.
The Sonnenberg Laboratory is supported by the National Institutes of Health (R01AI143842, R01AI123368, R01AI145989, R01AI162936, R21CA249284 and U01AI095608), the NIAID Mucosal Immunology Studies Team (MIST), the Crohns and Colitis Foundation, the Searle Scholars Program, the American Asthma Foundation Scholar Award, Pilot Project Funding from the Center for Advanced Digestive Care (CADC), an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund, a Wade F.B. Thompson/Cancer Research Institute (CRI) CLIP Investigator grant, the Meyer Cancer Center Collaborative Research Initiative, the Dalton Family Foundation, Linda and Glenn Greenberg, and the Roberts Institute for Research in IBD. Gregory F. Sonnenberg is a CRI Lloyd J. Old STAR. John Benji Grigg is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number F31AI138389-01A1. Support for human sample acquisition through the JRI IBD Live Cell Bank is offered by the JRI, Jill Roberts Center for IBD, Cure for IBD, the Rosanne H. Silbermann Foundation and Weill Cornell Medicine Division of Pediatric Gastroenterology and Nutrition.