” Were enthusiastic that these findings will lead to a much better understanding of prion and other neurodegenerative diseases, in addition to brand-new methods for treating them,” says study senior author Sandra Encalada, PhD, Arlene and Arnold Goldstein Associate Professor in the Department of Molecular Medicine at Scripps Research.
The researchers in their research study carefully observed mutant, disease-causing copies of the prion-disease protein PrP forming big aggregates in the axons of neurons, but not in the neurons main cell bodies. The development of these aggregates was followed by signs of axon dysfunction and eventually neuronal death. The researchers discovered evidence that nerve cells waste-disposal procedures generally are able to handle such aggregates when they are within or near neurons main cell bodies, but are much less able to do so when the aggregates build up far out within axons.
The scientists also determined a complex of crucial proteins as being accountable for steering PrP into axons and causing aggregation connected with big axonal swellings. They demonstrated that by silencing any one of these proteins they could hinder the aggregates from forming and protect the nerve cells from damage and death.
Vulnerable axons.
Since these aggregates grow by a chain-reaction process that draws in healthy copies of PrP, they can transmit CJD in uncommon cases– for example, throughout corneal transplant surgical treatment– from one individual to another. About 15 percent of cases are genetic, caused by mutations that make PrP more most likely to aggregate.
In the research study, Encaladas team utilized mouse brain cells consisting of mutant PrP, in addition to microscopic motion-picture strategies, to study the preliminary accumulation of PrP aggregates in axons. A nerve cells axon is often long in relation to its primary body– the soma– and has actually been discovered to be uniquely susceptible to disruptions of its delicate systems for carrying essential particles and eliminating waste.
PrPs common function in nerve cells has never been clear, however the protein seems usually secreted, by means of sac-like containers called vesicles, from the soma and the axon, where it in some cases goes back to be recycled or degraded as waste. The scientists found in their experiments that mutant PrP produced in the soma is also largely encapsulated in blisters that get moved into the axon along trains called microtubules.
This movement includes a rather intricate vesicle trafficking system, and the scientists observed that this system shunts much of the PrP far out into axons, where PrP-containing blisters merge and gather. Mutant PrP in this scenario forms big aggregates– Encalada calls them endoggresomes– that axons cant get rid of. The aggregates lead to axonal swellings, and other signs of dysfunction including minimized neuronal calcium signaling, and eventually a much faster neuronal death rate compared to nerve cells with regular PrP.
The researchers likewise found a way of countering endoggresomes development. They determined four proteins, Arl8, kinesin-1, Vps41, and SKIP, that are accountable for directing PrP-containing blisters into axons, carrying them far out into the soma, and combining them with other PrP-containing vesicles to activate aggregate formation. When they silenced any of these proteins, far fewer PrP-containing blisters got in axons, the axons revealed few or no indications of aggregation, and the nerve cells operated normally or nearly typically and endured simply as well as regular brain cells.
The outcomes indicate the alluring possibility that prion illness, and perhaps lots of other protein-aggregate illness of the brain, can be avoided or dealt with by disrupting a minimum of transiently the trafficking process that brings vesicle-encapsulated, aggregate-prone proteins out into axons.
” Were very passionate about discovering particles that can inhibit this aggregate-forming pathway and studying the impacts of such inhibitors in animal designs of prion and other neurodegenerative illness,” Encalada states.
Referral: “Endosomal sorting drives the development of axonal prion protein endoggresomes” by Romain Chassefeyre, Tai Chaiamarit, Adriaan Verhelle, Sammy Weiser Novak, Leonardo R. Andrade, André D. G. Leitão, Uri Manor and Sandra E. Encalada, 22 December 2021, Science.DOI: 10.1126/ sciadv.abg3693.
” Endosomal Sorting Drives the Formation of Axonal Prion Protein Endoggresomes” was co-authored by Romain Chassefeyre, Tai Chaiamarit, Adriaan Verhelle, André Leitão and Sandra Encalada, all of Scripps Research; and Sammy Weiser Novak, Leonardo Andrade and Uri Manor, of the Salk Institute for Biological Studies.
The research study was moneyed by the National Institutes of Health (R01AG049483) and others.
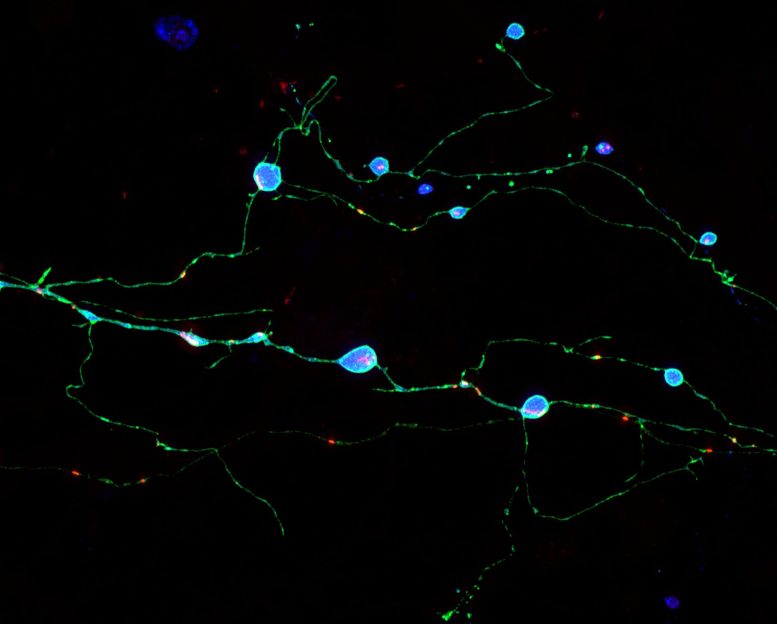
Neurons grown in culture expressing a mutant prion protein (cyan) that cause prion illness in people. These nerve cells show inflamed axons which contain harmful mutant prion protein aggregates. Chassefeyre et al. determined genes that account for the development of these aggregates and revealed that decreasing their function can hinder aggregate formation and prevent neuronal dysfunction. Credit: Adriaan Verhelle and Yin Wu (Scripps Research).
Scripps Research Discovery Illuminates How Brain Cells Die in Prion Diseases.
Researchers demonstrate how poisonous aggregates are formed inside brain cells, and how to block the cell-killing process– which might also be at operate in Alzheimers and other neurodegenerative diseases.
Prion diseases, such as Creutzfeldt-Jakob Disease (CJD), are fast-moving, deadly dementia syndromes connected with the development of aggregates of the prion protein, PrP. How these aggregates form within and eliminate brain cells has never ever been completely comprehended, but a new research study from researchers at Scripps Research recommends that the aggregates eliminate neurons by damaging their axons, the narrow nerve fibers through which they send out signals to other neurons.
The accumulation of protein aggregates in axons, together with axonal swellings and other signs of dysfunction, are also early functions of other neurodegenerative disorders including Alzheimers and Parkinsons illness. The discovery of how these prion aggregates form in axons and how to prevent them, reported in Science Advances, might eventually have a significance that goes far beyond prion illness.
These nerve cells show inflamed axons that consist of poisonous mutant prion protein aggregates. Chassefeyre et al. determined genes that account for the formation of these aggregates and revealed that minimizing their function can hinder aggregate development and prevent neuronal dysfunction. The scientists in their research study closely observed mutant, disease-causing copies of the prion-disease protein PrP forming big aggregates in the axons of nerve cells, but not in the nerve cells main cell bodies. The researchers discovered proof that nerve cells waste-disposal processes usually are able to cope with such aggregates when they are within or close to nerve cells primary cell bodies, however are much less able to do so when the aggregates build up far out within axons.
They identified four proteins, Arl8, kinesin-1, Vps41, and SKIP, that are accountable for directing PrP-containing vesicles into axons, bring them far out into the soma, and combining them with other PrP-containing vesicles to set off aggregate development.