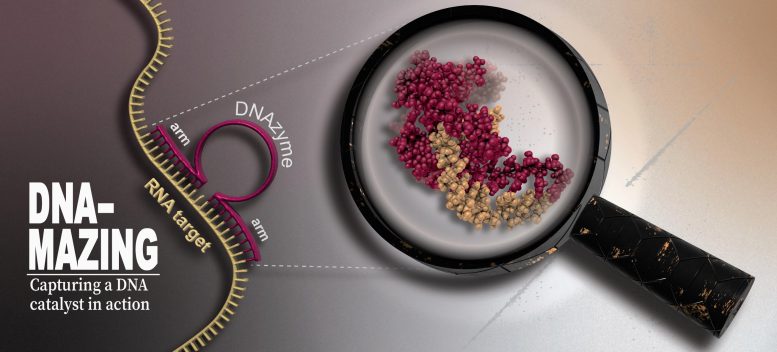
A DNAzyme (red) utilizes its binding arms to dock at a particular area on an RNA strand (yellow) and after that cleaves it at its core. High-resolution, real-time NMR, Electron Paramagnetic Resonance and Fluorescence Spectroscopy, along with Molecular Dynamics Simulations were used to identify the structure and catalytic mechanisms of the DNAzyme. Credit: HHU/Manuel Etzkorn
DNAzymes are accuracy biocatalysts that ruin undesirable RNA particles. Major challenges to their use in medicine remain. Together with Jülich Research Centre (FZJ) and the University of Bonn, a research study team from Heinrich Heine University Düsseldorf (HHU) has examined with atomic resolution how DNAzymes operate in genuine time. They have now presented these crucial essential findings and their application in the renowned journal Nature.
DNAzymes– a word made up of DNA and enzyme– are catalytically active DNA sequences. They consist of a catalytic core making up around 15 nucleic acids flanked by brief binding arms on the right- and left-hand sides, each with around ten nucleic acids. While the series of the core is repaired, the binding arms can be modified to specifically match essentially any RNA target series.
The goal is to target unwanted RNA molecules of infections, cancer, or harmed nerve cells, utilizing DNAzymes to attack and destroy them. The DNAzyme docks precisely to the coordinating position and the core cleaves the RNA particle, the pieces of which are then quickly deteriorated in the cell.
The healing benefits are obvious: Unwanted RNA can be damaged precisely, while other, useful RNA strands in a cell remain untouched. In some infections like SARS-CoV2 and Ebola, the hereditary material is coded on an RNA molecule. Like healthy cells, cancer cells utilize so-called messenger RNA (mRNA) to copy the blueprints for proteins from their DNA and transfer them to the particle factories. The mRNA series in cancer cells is frequently slightly various to that of healthy cells or present in various amounts, implying that DNAzymes can particularly assault cancer cells while sparing others.
“In a test tube, the DNAzymes are extremely efficient at damaging the RNA molecules, but this seldom occurs in a cell. Without an essential understanding of how they operate, it is extremely tough to establish improved DNAzyme variations that can accomplish their work in cells.
In their research study, the authors from HHU and a group from Jülich Research Centre (FZJ), the University of Bonn and a Swiss company looked for to understand how the system as an entire functions dynamically, what actions happen in the binding and cleaving process and what cofactors support the response.
The scientists observed the procedures at atomic resolution and in part in real time utilizing high-resolution nuclear magnetic resonance (NMR) spectroscopy. This allowed them to portray the three-dimensional atomic arrangement presumed by the DNAzyme to bind to and cleave the RNA: The core twists around the RNA hair in a highly efficient method, cleaving it into 2 pieces in several intermediate actions. After cleaving, the DNAzyme releases the fragments and can bind again somewhere else.
Teacher Dr. Holger Gohlke from the HHU Chair of Pharmaceutical and Medicinal Chemistry and the Institute of Bio- and Geosciences at FZJ, whose group carried out molecular characteristics simulations on the DNAzyme/RNA complex, adds: “In the finest sense of integrative modelling, we were able to put forward a plausible RNA cleaving system at atomic level and supply information on RNA base choice at the cleavage website.”
Jan Borggräfe, doctoral researcher in Etzkorns working group and lead author of the study, describes why the DNAzymes do not work well in cells: “We established that magnesium, as an essential cofactor, plays various vital functions in the system, but that it binds reasonably inadequately and only briefly to the DNAzyme. There are other components in the cell with a greater affinity for magnesium that “take” the magnesium from the DNAzyme so to speak.”
The next step is to perform structural investigations into cell cultures and organoids. The objective for restorative applications is to improve the magnesium affinity of the DNAzymes through targeted modifications in order to increase their activity in biological tissue.
Dr. Etzkorn specifies a more area of application: “The focus of our Institute rests on research study into neurodegenerative diseases, where we likewise see great capacity for DNAzymes. In the case of Parkinsons illness, they may under specific scenarios have the ability to destroy the mRNA sequence that drives the production of alpha-synuclein which, in large amounts, can promote neurotoxic procedures.” DNAzymes could also provide increase to a new class of prescription antibiotics.
Teacher Dr. Dieter Willbold, Director of the HHU Institute of Physical Biology and the FZJ Institute for Structural Biochemistry, includes: “The study is yet another example of how basic research study in structural biology can supply important contributions to ground-breaking biomedical advances. The new flagship of the biomolecular NMR center, a 1.2 GHz NMR gadget, has actually already contributed to this success.” The device at the Biomolecular NMR Center, which is jointly run by HHU and FZJ, is one of the most effective systems in the world and supplies unique insights into the structure and performance of the foundation of life. A short documentary movie about the complex installation of the device can be discovered listed below:
A DNAzyme (red) uses its binding arms to dock at a particular location on an RNA strand (yellow) and then cleaves it at its core. DNAzymes are precision biocatalysts that damage undesirable RNA molecules. The aim is to target undesirable RNA particles of viruses, cancer, or damaged nerve cells, utilizing DNAzymes to attack and ruin them. The DNAzyme docks specifically to the matching position and the core cleaves the RNA particle, the fragments of which are then quickly degraded in the cell. “In a test tube, the DNAzymes are extremely reliable at damaging the RNA particles, but this hardly ever happens in a cell.
Reference: “Time-resolved structural analysis of an RNA-cleaving DNA catalyst” by Jan Borggräfe, Julian Victor, Hannah Rosenbach, Aldino Viegas, Christoph G. W. Gertzen, Christine Wuebben, Helena Kovacs, Mohanraj Gopalswamy, Detlev Riesner, Gerhard Steger, Olav Schiemann, Holger Gohlke, Ingrid Span and Manuel Etzkorn, 23 December 2021, Nature.DOI: 10.1038/ s41586-021-04225-4.