In their research study, which released just recently in the journal Cell, Anand and his associates investigated the interactions in between human monoclonal antibody (HMAb) C10 and 2 disease-causing viruses: Zika and dengue. The HMAb C10 antibodies they utilized had actually formerly been isolated from clients infected with dengue virus and also had been shown to reduce the effects of Zika infection.
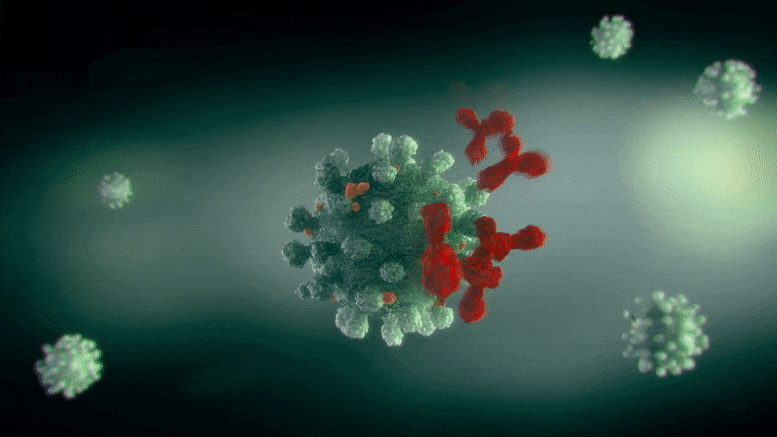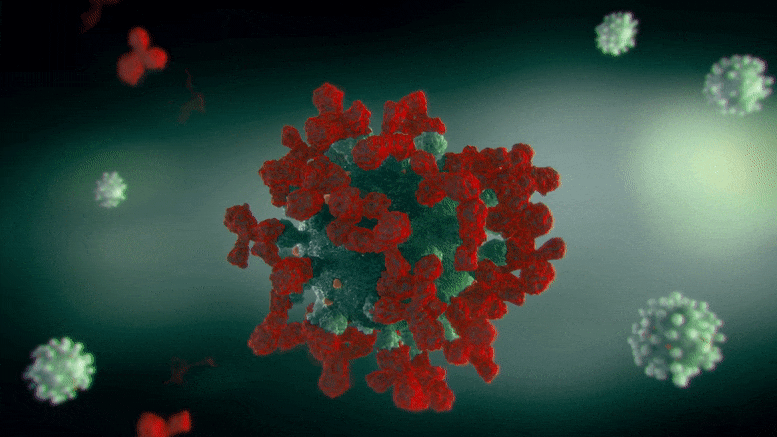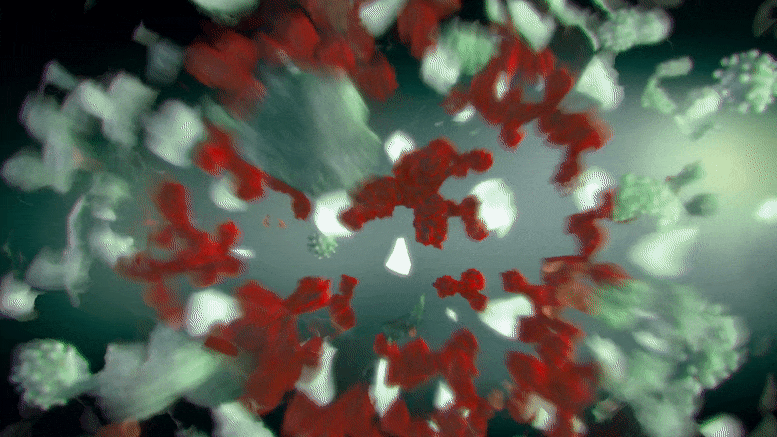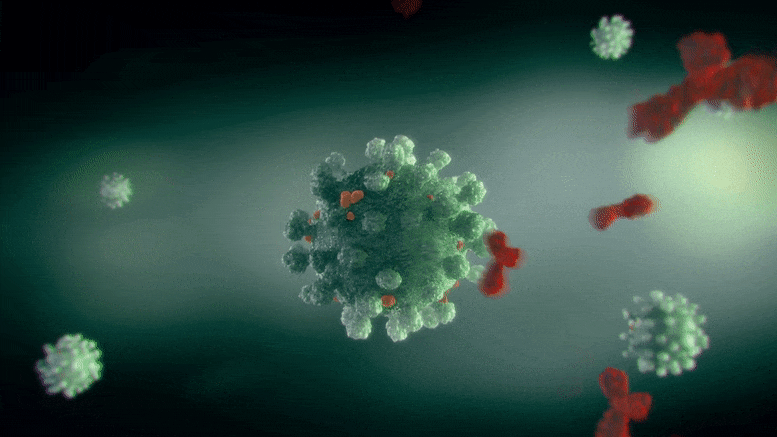
Researchers found that the same type of antibody can neutralize Zika and dengue viruses in 2 different methods– one where it binds to the infection and deactivates it (left), which is the standard method we think of antibody activity, and the other where it burrows in and distorts the virus (right). Credit: Ganesh Anand, Penn State
The researchers used a combination of techniques, including cryogenic electron microscopy (cryo-EM) to picture the viruses and hydrogen/deuterium exchange mass spectrometry (HDXMS) to comprehend their movement.
” Cryo-EM includes flash-freezing a solution including molecules of interest and then targeting them with electrons to produce numerous pictures of individual molecules in various orientations,” described Anand. “These images are then incorporated into one photo of what the particle looks like. The method offers far more precise photos of molecules than other forms of microscopy.”
To document the results of antibodies on Zika and dengue infections, the team gathered cryo-EM snapshots of the viruses under conditions of increasing concentrations of antibodies.
In parallel, the team used HDXMS, a strategy in which particles of interest– in this case Zika and dengue virus, in addition to HMAb C10 antibodies– are immersed in heavy water. Heavy water, Anand discussed, has had its hydrogen atoms replaced with deuterium, hydrogens heavier isotopic cousin.
” When you submerge a virus in heavy water, the hydrogen atoms on the surface area of the virus exchange with deuterium,” he stated. By doing this, we observed that dengue virus, however not Zika infection, became heavier with deuterium as more antibodies were included to the solution.
In contrast, Zika virus did not become heavier when positioned in heavy water, recommending that its surface, while completely inhabited by antibodies, is not distorted by the antibodies.
Anand explained that by integrating cryo-EM and HDXMS, the group had the ability to get a thorough photo of what occurs when antibodies connect to Zika and dengue infections.
” Its like those animation flipbooks, where each page has a slightly various image, and when you flip through the book, you see a short motion picture,” he said. This complementary set of tools enabled us to understand how one type of antibody differentially impacts two types of viruses.”
He noted that the reality that the more antibodies they included, the more distorted the dengue infection particles became, recommends that stoichiometry– the relationship in between the amounts of the reactants and the products prior to, throughout, and after a chemical reaction– matters.
” Its not sufficient to just have antibodies present,” he said. “How much antibody you include determines the level of neutralization.”
The team discovered that at saturating conditions, in which antibodies were included at high sufficient concentrations to fill all the available binding places on the dengue infections, 60% of the virus surfaces ended up being distorted. This distortion was enough to secure the cells from infection.
” If you have adequate antibodies, they will misshape the infection particle enough so that its preemptively destabilized prior to it even reaches its target cells,” Anand stated.
When the researchers bred the antibody-bound dengue infections with BHK-21 cells, a cell line from the kidneys of infant hamsters that is frequently utilized in viral infection research, they found that 50% -70% less cells were contaminated.
Anand discussed that with some viruses, consisting of Zika, antibodies work by jamming the exits so the passenger can not get out of the cars and truck.
” We have actually discovered a new system in dengue infection whereby antibodies essentially total the automobile so it can not even travel to a cell,” he said.
How are the antibodies misshaping the dengue infection particles?
Anand explained that contrary to the now-familiar SARS-CoV-2, which has spike proteins protruding in all directions, the surfaces of both Zika and dengue are smoother with peaks and valleys.
Anand noted that for dengue virus, antibodies specifically prefer binding the peaks called five-fold vertices. When all the proteins on the five-fold vertices have actually been bound, antibodies will rely on their second-favorite peaks– the three-fold vertices. Lastly, they are entrusted only the two-fold vertices.
“We discovered that when the five- and three-fold vertices have been totally bound with antibodies, if we include more antibodies to the service, the infection starts to shudder. As a result, these antibodies end up burrowing into the infection rather than binding onto the two-fold vertices, and we believe its this digging into the infection particle that causes the virus to shake and misshape and ultimately end up being nonfunctional.”
What is the difference in between Zika and dengue?
Anand explained that Zika is a far more stable, less dynamic infection than dengue, which has a lot of moving parts.
” Dengue and Zika look similar but each one has a different offer. Dengue might have developed as a more mobile virus as a way of avoiding being caught by antibodies. Its moving parts confuse and throw off the body immune system. Unfortunately for dengue, antibodies have evolved a way around this by burrowing into the virus and distorting it.”
It appears, he said, that the very same type of antibody can neutralize Zika and dengue in 2 different ways– one where it binds to the infection and deactivates it, which is the conventional way we consider antibody activity, and the other where it burrows in and distorts the virus.
What about other infections?
Anand stated the distortion method his group discovered might be used by antibodies when they are faced with other types of viruses.
” Dengue is just a design virus that we used in our experiments, however we believe this preemptive destabilization strategy might be broadly appropriate to any infection,” he said. “It might be that the antibodies first attempt to neutralize infections through the barrier approach and if they are unsuccessful, they resort to the distortion method.”
Are there any prospective applications of the findings?
The findings might be useful in developing therapeutic antibodies, Anand said.
” HMAb C10 antibodies are particular to dengue and Zika infections, and occur to be capable of reducing the effects of Zika and dengue viruses in 2 different methods,” he stated. “But you could possibly create rehabs with the very same abilities for dealing with other illness, such as COVID-19. By creating a healing with antibodies that can both block and distort infections, we can perhaps accomplish higher neutralization.”
He included, “You dont want to await an infection to reach its target tissue, so if you can present such a therapeutic cocktail as a nasal spray where the virus very first goes into the body, you can prevent it from even entering the system. By doing this, you may even have the ability to use less antibody because our research study reveals that it takes less antibody to reduce the effects of a virus through the distortion approach. You can improve bang for the buck.”
In general, Anand worried that the significance of the research study is that it exposes an entirely brand-new strategy that some antibodies use to disable some viruses.
” Previously, all we knew about antibodies was that they bind and neutralize viruses,” he stated. “Now we understand that antibodies can neutralize infections in a minimum of two various ways, and possibly even more. This research unlocks to a whole brand-new avenue of exploration.”
Recommendation: “Human antibody C10 reduces the effects of by diminishing Zika but enhancing dengue infection dynamics” by Xin-Xiang Lim, Bo Shu, Shuijun Zhang, Aaron W.K. Tan, Thiam-Seng Ng, Xin-Ni Lim, Valerie S.-Y. Chew, Jian Shi, Gavin R. Screaton, Shee-Mei Lok and Ganesh S. Anand, 30 November 2021, Cell.DOI: 10.1016/ j.cell.2021.11.009.
Other authors on the paper consist of Xin-Xiang Lim, graduate student; Jian Shi, manager, Cryo-EM Facility; and Shee-Mei Lok, teacher, National University of Singapore. Co-authors likewise include Bo Shu, research fellow; Shuijun Zhang, assistant teacher; Aaron W.K. Tan, college student; Thiam-Seng Ng, college student; Xin-Ni Lim, graduate trainee; and Valerie Chew, assistant teacher, Duke-National University of Singapore Medical School. Gavin R. Screaton, head of the Medical Sciences Division, Oxford University, likewise is an author.
This research was supported by the National Research Foundation of Singapore, the Ministry of Health of Singapore, and by Penn State.
It is commonly comprehended that antibodies reduce the effects of infections by latching onto their surfaces and blocking them from contaminating host cells. However new research study exposes that this barrier technique isnt the only method that antibodies disable viruses. An international team of scientists led by Penn State has discovered that antibodies also distort infections, consequently avoiding them from appropriately attaching to and entering cells.
” Everybody thinks of antibodies as binding to viruses and obstructing them from going into cells– basically locking them down,” stated Ganesh Anand, associate teacher of chemistry at Penn State. “But our research reveals for the very first time that antibodies may likewise physically misshape infections, so they are not able to effectively connect to and contaminate host cells.”
” When you immerse an infection in heavy water, the hydrogen atoms on the surface of the virus exchange with deuterium,” he stated. By doing this, we observed that dengue infection, but not Zika infection, ended up being much heavier with deuterium as more antibodies were added to the service. As an outcome, these antibodies end up burrowing into the virus rather than binding onto the two-fold vertices, and we believe its this digging into the infection particle that causes the virus to shake and misshape and eventually become nonfunctional.”
” HMAb C10 antibodies are specific to dengue and Zika viruses, and occur to be capable of reducing the effects of Zika and dengue viruses in 2 various ways,” he stated. He added, “You dont want to wait for a virus to reach its target tissue, so if you can introduce such a restorative cocktail as a nasal spray where the virus very first enters the body, you can avoid it from even going into the system.