” However, while investigations of how the FOP anomaly modifies the policy of cell fate decisions have been thoroughly pursued in the last few years, little attention has been paid to the results of the genetic mutation on muscle and its influence on the cells that fix muscle injuries,” Shore stated. “We were encouraged that pursuing research study in this area could offer ideas not just for avoiding extra bone development but also for improving muscle function and regeneration, bringing new clarity to FOP as a whole.”
They focused on two particular types of muscle tissue stem cells: fibro-adipogenetic progenitors (FAPs) and muscle stem cells (MuSCs). Injured tissue reacts by an expansion of FAP cells, which are designated to recruit muscle stem cells that will regrow the damaged muscle tissue. At the very same time, MuSCs shift towards a more fully grown, differentiated state, called muscle fiber, essential to organized movement of our muscles.
In the mice with the ACVR1 anomaly that Mourkioti, Shore, and their co-authors studied, apoptosis– the process through which FAP cells pass away as a part of appropriate muscle regeneration– had actually slowed considerably, resulting in a high existence of FAPs past their usual life expectancy. This changed their balance with the MuSCs. The hurt tissue likewise revealed a reduced capacity for muscle stem cell maturation and, as a result, muscle fibers were considerably smaller in mice carrying the ACVR1 anomaly compared to muscle fibers in mice without the mutation.
” The prolonged perseverance of unhealthy FAPs within the regenerating muscle contributes to the transformed muscle environment in FOP, which decreases muscle regeneration and allows the over-abundant FAPs to add to the formation of extra-skeletal bone,” Mourkioti stated. “This provides a totally brand-new point of view on how excess extra-skeletal bone is formed– and how it might be avoided.”
The present targets for dealing with FOP focus on slowing extra-skeletal bone growth. This research may offer a pivotal brand-new instructions. “We propose that healing interventions must think about promoting the restoring potential of muscles together with the decrease of ectopic bone formation,” Shore and Mourkioti wrote. “By addressing both stem cell populations and their roles in the origin of FOP, there is the possibility of greatly boosted treatments.”
This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01‐AR041916‐15S1, F31-AR069982), the National Institutes of Health (R01‐AR071399, NIH P30-AR069619), and the International Fibrodysplasia Ossificans Progressiva Association (IFOPA).
Other authors in the study include Alexandra Stanley, Elisia Tichy, Jacob Kocan and Douglas Roberts.
Recommendation: “Dynamics of skeletal muscle-resident stem cells throughout myogenesis in fibrodysplasia ossificans progressive” by Alexandra Stanley, Elisia D. Tichy, Jacob Kocan, Douglas W. Roberts, Eileen M. Shore and Foteini Mourkioti, 14 January 2022, npj Regenerative Medicine.DOI: 10.1038/ s41536-021-00201-8.
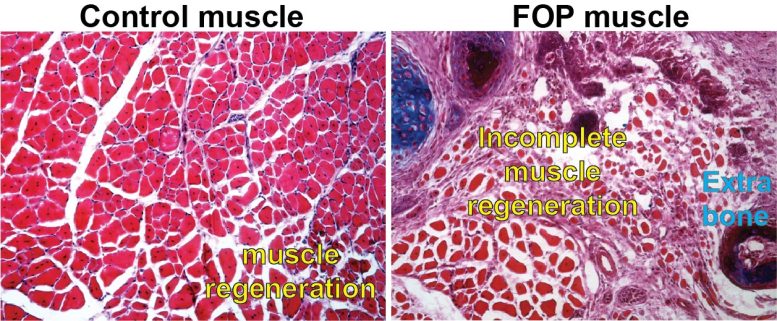
A picture of a control cell with normal muscle regeneration compared to a cell with the exact same hereditary anomaly that individuals with FOP have. Credit: Courtesy of Penn Medicine
Fibrodysplasia ossificans progressiva (FOP) is a rare illness characterized by substantial bone development outside of the regular skeleton that pre-empts the bodys regular actions to even small injuries. Inefficient and impaired muscle tissue regeneration appears to open the door for unwanted bone to form in areas where new muscle need to take place after injuries.
” While we have made fantastic strides towards much better understanding this illness, this work reveals how fundamental biology can offer fantastic insights into suitable regenerative medication therapies,” stated the research studys lead author, Foteini Mourkioti, PhD, an assistant teacher of Orthopaedic Surgery and Cell and Developmental Biology, in addition to the co-director of the Penn Institute for Regenerative Medicine, Musculoskeletal Program. “From the laboratory, were now able to reveal that there is capacity for a whole brand-new realm of treatments for clients with this devastating condition.”
About 15 years earlier, researchers at Penn– including this studys co-author, Eileen Shore, PhD, a professor in Orthopaedic Surgery and Genetics and the co-director of the Center for Research in FOP and Related Disorders– discovered that a mutation in the ACVR1 gene was accountable for FOP. Because study, the group discovered that the mutation changed cells within muscles and connective tissues, misdirecting cells within the tissue to act like bone cells, leading to unnecessary and brand-new extra-skeletal bone within the body.
Inefficient and impaired muscle tissue regeneration appears to open the door for undesirable bone to form in locations where new muscle ought to occur after injuries. They focused on 2 particular types of muscle tissue stem cells: fibro-adipogenetic progenitors (FAPs) and muscle stem cells (MuSCs). Injured tissue responds by an expansion of FAP cells, which are appointed to recruit muscle stem cells that will restore the damaged muscle tissue. At the same time, MuSCs transition toward a more mature, distinguished state, called muscle fiber, important to arranged motion of our muscles.
The hurt tissue also revealed a reduced capacity for muscle stem cell maturation and, as an outcome, muscle fibers were considerably smaller in mice carrying the ACVR1 anomaly compared to muscle fibers in mice without the mutation.