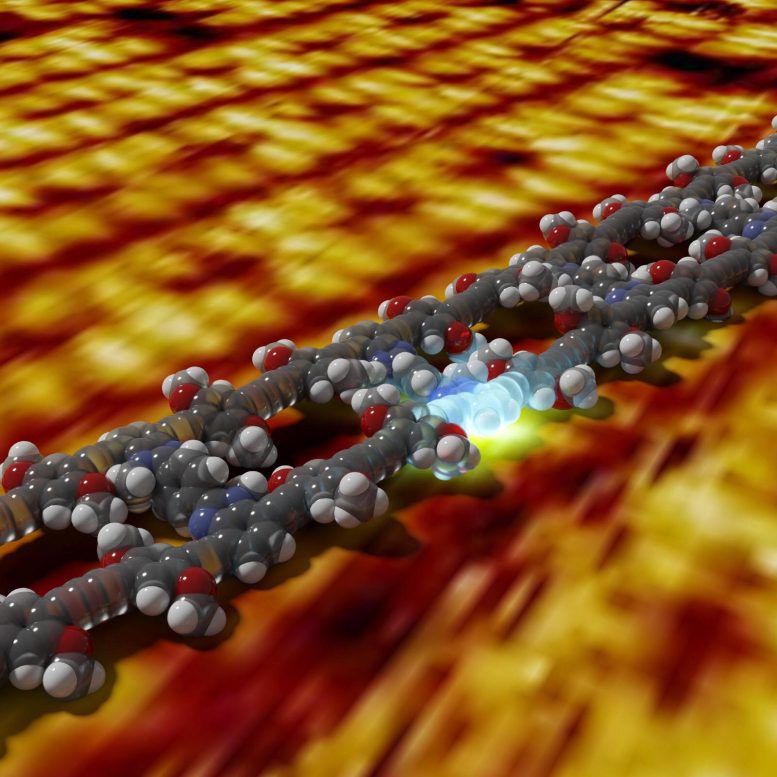
A packet of energy is created on a molecular ladder by the absorption of light. The background image reveals a genuine measurement of the ladder structure with a scanning tunneling microscopic lense. Credit: Joshua Bahr and Tristan Keller
Researchers produce novel molecules that serve as ziplines for energy.
In the 19th century, the clinical community puzzled over how the atoms in the strange substance benzene were organized. This “fragrant” molecule soon proved to have a remarkably simple structure: It included six carbon and six hydrogen atoms. How could these twelve atoms organize themselves in space to form a chemically stable item? The chemist Friedrich August Kekulé, later on professor at the University of Bonn, brought light into the darkness. Legend has it that he sat dozing by the fireplace in the winter of 1861. Kekulé unexpectedly had a vision of a snake devouring its own tail. He recognized that the carbon atoms of benzene should be organized in a circle, comparable to a small wagon wheel.
” This dream ultimately laid the foundation for the huge expansion of the chemical industry towards completion of the 19th century,” says Prof. Sigurd Höger of the Kekulé Institute of Organic Chemistry and Biochemistry at the University of Bonn, who belongs to the Transdisciplinary Research Area “Building Blocks of Matter and Fundamental Interactions” at the University of Bonn. Benzene is an important structure block for plastics, paints, and pharmaceuticals.
A package of energy is created on a molecular ladder by the absorption of light. Kekulés followers at the University of Bonn had actually long been dreaming of particles in the shape of a ladder, consisting of hundreds of benzene rings. The scientists from the Kekulé Institute and the Mulliken Center for Theoretical Chemistry at the University of Bonn, together with a team led by Prof. John Lupton from the Institute of Experimental and Applied Physics at the University of Regensburg, have actually now built such a molecular ladder. In this way, in addition to the polymer with a single conjugated rail, the group got a polymer with two conjugated rails– the stiff “ladder”. The chemists also validated the shape and amazing rigidity of the ladders– compared to the snakes– through substantial computer system simulations using an unique theory that forecasts the individual motions of all atoms within the particle.
Computer system simulation of the dynamics of a molecular “snake” and a “ladder.” The snake folds quickly while the ladder remains rigid over the time of the simulation. Credit: Sebastian Spicher
Hundreds of benzene rings in the shape of a ladder
Kekulés successors at the University of Bonn had actually long been dreaming of particles in the shape of a ladder, consisting of hundreds of benzene rings. The scientists from the Kekulé Institute and the Mulliken Center for Theoretical Chemistry at the University of Bonn, together with a group led by Prof. John Lupton from the Institute of Experimental and Applied Physics at the University of Regensburg, have now built such a molecular ladder.
In this way, in addition to the polymer with a single conjugated rail, the team acquired a polymer with two conjugated rails– the stiff “ladder”. Both polymers were of equal length and could now be compared to each other: How would turning a snake into a ladder impact the materials residential or commercial properties?
The researchers took a look at the structure using a scanning tunneling microscope. The tiny molecular ladder is one nanometer (a millionth of a millimeter) high, two nanometers wide and one hundred nanometers long. The chemists also confirmed the shape and amazing rigidness of the ladders– compared to the snakes– through extensive computer simulations utilizing a novel theory that forecasts the individual motions of all atoms within the molecule.
Prospective foundation for electronics
” The ladder structure is maintained not only when the molecules are placed on a surface area, but likewise when they are dissolved in a liquid,” states Prof. Lupton of the University of Regensburg. This function, he includes, allows energy to move along the molecule in area, supplying a potential building block for optical networks, sensing units and circuits.
In principle, such polymers carry out electrical currents and can be utilized to make new screens based on organic light-emitting diodes (OLEDs), or to transform light into electricity in a solar cell. When light falls onto such a molecule, it is absorbed and produces a little package of energy. The researchers had the ability to observe how these packages moved along the ladder essentially unimpeded, as if on a zipline. The open snake-like polymers, on the other hand, do not show this impact. Their homes are similar to those of conventional polymer molecules: the packages slide along the “snakes” and lose energy.
Kekulés shattered dream
” While old Kekulé saw the single particle as a ring, he definitely never ever dreamed that there would one day be huge particles of such rigidity that they are not able to bite their own tails,” says Höger, summing up the outcome with a wink.
Referral: “Nanoscale π-conjugated ladders” by Stefanie A. Meißner, Theresa Eder, Tristan J. Keller, David A. Hofmeister, Sebastian Spicher, Stefan-S. Jester, Jan Vogelsang, Stefan Grimme, John M. Lupton and Sigurd Höger, 16 November 2021, Nature Communications.DOI: 10.1038/ s41467-021-26688-9.