The cage was crafted with amino acid replacements by presenting site-specific anomalies that enabled more IrCp * uptake. Credit: Takafumi Ueno from Tokyo Institute of Technology
An unique hybrid ferritin nanocage with histidine residues reveals 1.5 times higher metal ion uptake and enhanced catalytic performance for alcohol production, find scientists from Tokyo Tech in a brand-new research study. Their findings recommend that hybrid bio-nanocages could effectively catalyze responses to yield industrially important products.
Biological polymers can spontaneously self-assemble into intricate structures that look like vessels or cages, but are much smaller, and are referred to as “nano-cages.” These structures can accommodate a large range of particles inside them that behave as “guests.” One popular example is the “ferritin nanocage,” which is formed by the self-assembly of 24 subunits into the protein ferritin and can confine metal ions that are essential drivers. With the help of these metal ions, a catalytic response converts any substrate into a product. Commonly known, the ferritin cages possible applications in market have yet to be fully checked out.
So far, most efforts to increase metal ion uptake in ferritin have actually led to cages with low stability. To make the “visitor” sit well within the cage, effective design is the key. Keeping that in mind, a team of scientists led by Prof. Takafumi Ueno, from Tokyo Institute of Technology, Japan (Tokyo Tech), presented site-specific anomalies at the core of the ferritin nanocage and increased its uptake of iridium complex (IrCp *). Their findings are published in Angewandte Chemie. Iridium is an essential catalyst in the alcohol production path and is utilized commercially in the pharmaceutical, food, and chemical markets.
Commonly known, the ferritin cages potential applications in industry have yet to be totally checked out.
Therefore far, the majority of efforts to increase metal ion uptake in ferritin have resulted in cages with low stability. Prof. Ueno describes, “Based on previous literature, we knew that the presence of coordination amino acids in the cage improve iridium activity, and that replacing these amino acids with appropriate residues might minimize the problem. The authors utilized the amino acid histidine to replace two residues, arginine, and aspartic acid of the routine (wild type) ferritin cages and produce the mutants R52H and D38H. According to Prof. Ueno, “This implies that it is not only the presence of the histidine residue however likewise its position that is crucial to figure out uptake efficiency in the cage.”
The nanocage functions as a hybrid bio-catalyst throughout the conversion of substrates to alcohols with high uniqueness. Credit: Takafumi Ueno from Tokyo Institute of Technology
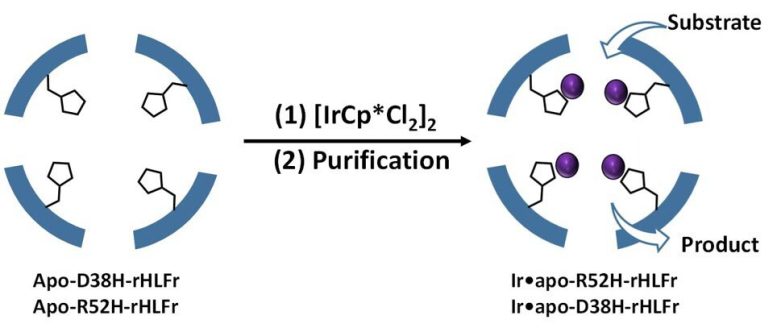
Prof. Ueno explains, “Based on previous literature, we understood that the presence of coordination amino acids in the cage enhance iridium activity, and that substituting these amino acids with proper residues might alleviate the problem. Considering that iridium complex behaves as a driver, coordination residues would do the task.” The authors used the amino acid histidine to change two residues, arginine, and aspartic acid of the routine (wild type) ferritin cages and produce the mutants R52H and D38H. Incredibly, the assembly structure or cage size were not impacted by these modifications.
Next, they included IrCp * to the mutants and discovered that R52H was able to embed 1.5 times more iridium atoms than the wild type cage (Figure 1). But, what struck them was the D38H mutant, which acted precisely like the wild type! So, why didnt both mutations have the very same result? According to Prof. Ueno, “This implies that it is not only the existence of the histidine residue however likewise its position that is crucial to determine uptake effectiveness in the cage.”
Utilizing the brand-new catalytic cages, the researchers had the ability to achieve alcohol production rates as high as 88%. Evidently, the anomalies preferred a structural re-arrangement of the reaction components, which boosted the conversion rate (Figure 2).
To understand how the substrate behaved inside the cage, the scientists used simulations in which the substrate particles might move freely within the nanocage. They observed some interactions in between the substrate and histidine in the R52H mutant, which were not present in the wild type cage, i.e., the substrate revealed preferential binding within the nanocage.
” These hybrid bio-nanocages were also discovered to be highly stable, suggesting that they could be utilized as feasible drivers in commercial applications,” concludes Prof. Ueno. The existing structure-based style of the metal ion binding site research study could be advanced to create unique ferritin mutants with selective uptake of specific guest molecules, for varied catalytic applications in the chemical and pharmaceutical industry.
Reference: “Controlled Uptake of an Iridium Complex inside Engineered apo-Ferritin Nanocages: Study of Structure and Catalysis” by Mohd Taher, Basudev Maity, Taiki Nakane, Satoshi Abe, Takafumi Ueno and Shyamalava Mazumdar, 10 January 2022, Angewandte Chemie.DOI: 10.1002/ anie.202116623.