” Understanding the molecular structure of the viral spike protein is very important as it will allow us to develop more efficient treatments versus Omicron and associated versions in the future,” said lead author Dr. Sriram Subramaniam (he/him), professor in UBCs department of biochemistry and molecular biology. “By examining the mechanisms by which the virus contaminates human cells, we can develop better treatments that interfere with that process and reduce the effects of the virus.”
The spike protein, which is located on the exterior of a coronavirus, enables SARS-CoV-2 to go into human cells. The Omicron variant has an unmatched 37 mutations on its spike protein– three to five times more than previous versions.
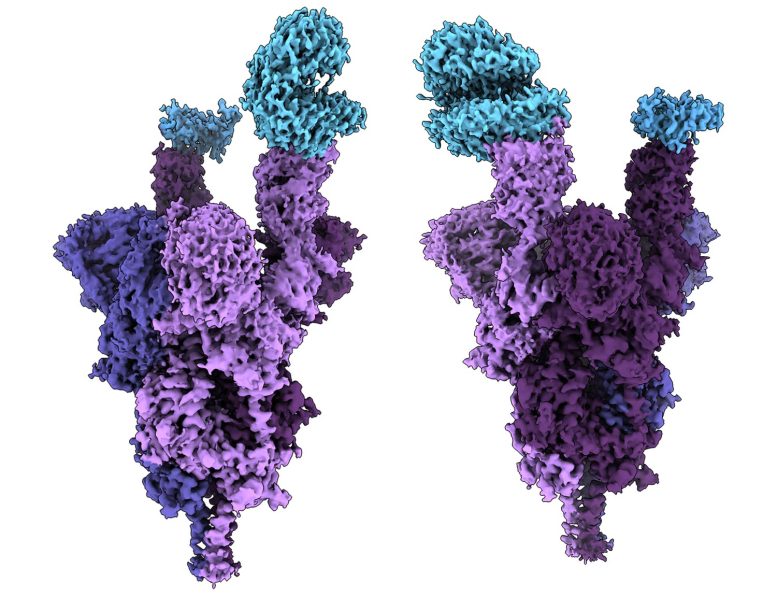
The structural analysis exposed that numerous mutations (S496, r498, and r493) produce new salt bridges and hydrogen bonds in between the spike protein and the human cell receptor called ACE2. The scientists concluded that these new bonds appear to increase binding affinity– how strongly the infection connects to human cells– while other mutations (K417N) reduce the strength of this bond.
” Overall, the findings reveal that Omicron has greater binding affinity than the original infection, with levels more equivalent to what we see with the Delta variation,” said Dr. Subramaniam. “It is amazing that the Omicron alternative progressed to maintain its ability to bind with human cells despite such substantial anomalies.”
The researchers carried out even more experiments showing that the Omicron spike protein exhibits increased antibody evasion. In contrast to previous variations, Omicron revealed measurable evasion from all six monoclonal antibodies checked, with complete escape from 5. The version also displayed increased evasion of antibodies collected from immunized people and unvaccinated COVID-19 patients.
” Notably, Omicron was less evasive of the immunity created by vaccines, compared to resistance from natural infection in unvaccinated clients. This suggests that vaccination remains our best defense,” stated Dr. Subramaniam.
Based upon the observed increase in binding affinity and antibody evasion, the scientists state that the spike protein mutations are likely contributing factors to the increased transmissibility of the Omicron version.
Next, Dr. Subramaniam states his research study group will utilize this knowledge to support the development of more effective treatments.
” An important focus for our group is to much better understand the binding of neutralizing antibodies and treatments that will work throughout the entire series of versions, and how those can be utilized to establish variant-resistant treatments.”
Referral: “SARS-CoV-2 Omicron variation: Antibody evasion and cryo-EM structure of spike protein– ACE2 complex” by Dhiraj Mannar, James W. Saville, Xing Zhu, Shanti S. Srivastava, Alison M. Berezuk, Katharine S. Tuttle, Ana Citlali Marquez, Inna Sekirov and Sriram Subramaniam, 20 January 2022, Science.DOI: 10.1126/ science.abn7760.
Atomic structure of the Omicron alternative spike protein (purple) bound with the human ACE2 receptor (blue). Credit: UBC Faculty of Medicine
Findings shed light on factors behind Omicrons increased transmissibility, consisting of strong antibody evasion and binding with human cells.
Researchers at UBCs faculty of medicine have actually carried out the worlds first molecular-level structural analysis of the Omicron variant spike protein. The findings were released on January 20, 2022, in Science.
The analysis– done at near atomic resolution using cryo-electron microscopy– exposes how the greatly mutated Omicron alternative attaches to and contaminates human cells.