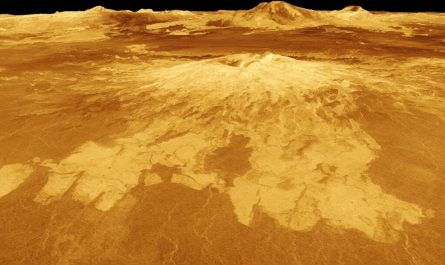
Schematic illustration of the generation of PROTAC infections (VP, viral protein; Ub, ubiquitin). Credit: Longlong Si
Utilizing attenuated, live infections as vaccines is a promising strategy to minimize the effect of viral contagious diseases, such as influenza.
Conventional live-attenuated virus vaccines have, however, typically been restricted due to suboptimal immunogenicity (the capability to invoke an immune response), security concerns, or cumbersome production processes and methods. Additionally, immune escape due to quick viral advancement poses a further difficulty for conventional influenza vaccines.
A team of scientists led by Prof. Longlong Si from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences has just recently proposed a new live-attenuated influenza vaccine method. Particularly, generating proteolysis-targeting chimeric (PROTAC) influenza An infection as a live-attenuated vaccine by using the endogenous ubiquitin-proteasome system of host cells to break down viral proteins.
The findings were released on July 4 in the journal Nature Biotechnology.
Because virus duplication depends on virally encoded proteins, control of viral protein stability by using the protein degradation machinery of the host cell may represent a prospective approach to change the viral life cycle on and off for vaccine development. Therefore, the scientists created proteolysis-targeting chimeric (PROTAC) viruses by fusing a conditionally detachable proteasome-targeting domain (PTD) to influenza viral proteins.
The PTD was developed to contain a proteasome-targeting peptide and a tobacco etch infection cleavage website (TEVcs) linker. It was used to selectively cause proteasomal destruction of viral proteins of interest; nevertheless, the TEVcs linker could be selectively cleaved by the tobacco etch virus protease (TEVp) to separate the viral proteins from the PTD, therefore sparing them from deterioration.
Accordingly, the researchers engineered the genome of influenza A viruses in TEVp-expressing steady cell lines engineered for virus production to introduce the conditionally detachable PTD, producing totally infective PROTAC viruses that were live-attenuated by the host protein deterioration machinery upon infection.
In mouse and ferret models, PROTAC viruses were sufficiently attenuated but able to generate robust and broad humoral, mucosal, and cellular immunity. As an outcome, they provided broad protection against homologous and heterologous infection challenges.
” This PROTAC vaccine innovation might also be helpful for creating live-attenuated vaccines against other kinds of pathogens,” said Prof. Si.
Reference: “Generation of a live attenuated influenza A vaccine by proteolysis targeting” by Longlong Si, Quan Shen, Jing Li, Li Chen, Jinying Shen, Xue Xiao, Haiqing Bai, Tang Feng, Adam Yongxin Ye, Le Li, Chunhe Zhang, Zhen Li, Ping Wang, Crystal Yuri Oh, Atiq Nurani, Siwen Niu, Chengxin Zhang, Xiaoqiong Wei, Wanqiong Yuan, Hao Liao, Xiaojie Huang, Ning Wang, Wen-xia Tian, Hongwei Tian, Li Li, Xiaoheng Liu and Roberto Plebani, 4 July 2022, Nature Biotechnology.DOI: 10.1038/ s41587-022-01381-4.