The leading cause of adult-onset macular degeneration (AMD) is due to a breakdown in the function of the retinal pigmented epithelium (RPE), a layer of tissue in between the photoreceptors that discover light and the choriocapillaris that provides nourishment to the retina. Recognizing the significance of RPE in the development of AMD, the authors of the research study focused on checking out the role of a transcription factor called LHX2. The groups analysis of mouse mutants showed that LHX2 plays a crucial role in RPE development.
Tearing down LHX2 activity in RPE stemmed from human stem cells, they found that the majority of impacted genes were down-regulated, suggesting that LHX2s role was most likely that of a transcriptional activator, binding to regulatory sites on the genome to increase the activity of other genes.
The authors found that a person affected gene, called OTX2, worked together with LHX2 to manage lots of genes in the RPE. By mapping the genomic websites that OTX2 and LHX2 might bind to, they showed that 68% of those that bound LHX2 were also bound by OTX2 (864 sites in all), recommending they most likely interact to promote the activity of a big suite of genes involved in RPE development and function.
A common technique for finding genes that might contribute to an illness is to perform a genome-wide association study (GWAS), which identifies genome sequence differences between individuals (called single nucleotide polymorphisms, or SNPs) that co-occur with the illness. Here, the authors compared their LHX2/OTX2 binding information to GWAS information in order to house in on variations that affected the binding of the transcription elements, and thus might contribute to disease.
One such binding site was located within the promoter area of a gene called TRPM1, which had actually been previously linked to AMD, and found that the sequence variant at that website altered the binding strength of LHX2; the so-called C variation bound it more strongly than the T version, and activity of the TRPM1 gene was higher when the C allele existed rather of the T allele.
The results of the research study show that the formerly understood increased risk of AMD from the alternative recognized in the GWAS was due to a decrease in the binding of the LHX2 transcription aspect to the TRPM1 gene promoter, with a consequent reduction in the activity of this gene. The gene encodes a membrane ion channel, and previous studies have shown that mutations in the gene likewise cause visual disability.
” Our research study exhibits how delineation of tissue-specific transcriptional regulators, their binding sites across the genome, and their downstream gene-regulatory networks can offer insights into an intricate diseases pathology,” the authors said.
Ashery-Padan includes, “The findings reveal a regulatory module consisting of LHX2 and OTX2 that manages the advancement and maintenance of the retinal pigmented epithelium, an important tissue of visual function. The genomic analyses further connect the genomic areas bound by the two developmental factors to the genetics of the common, multifactorial blinding disease age-related macular degeneration (AMD).”.
Reference: “The LHX2-OTX2 transcriptional regulative module controls retinal pigmented epithelium distinction and underlies hereditary risk for age-related macular degeneration” by Mazal Cohen-Gulkar, Ahuvit David, Naama Messika-Gold, Mai Eshel, Shai Ovadia, Nitay Zuk-Bar, Maria Idelson, Yamit Cohen-Tayar, Benjamin Reubinoff, Tamar Ziv, Meir Shamay, Ran Elkon and Ruth Ashery-Padan, 17 January 2023, PLOS Biology.DOI: 10.1371/ journal.pbio.3001924.
A typical method for finding genes that may contribute to a disease is to carry out a genome-wide association study (GWAS), which recognizes genome sequence differences in between people (called single nucleotide polymorphisms, or SNPs) that co-occur with the disease.
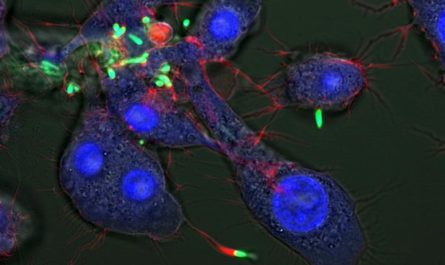
Composite of an embryonic mouse eye cup (E14.5) labeled with antibodies against the developmental transcription aspects Lhx2 (red) and Otx2 (green), and cultured human retinal pigmented epithelium (RPE) identified with antibodies against MITF (red) and ZO-1 (green). Credit: Images by Mazal Cohen-Gulkar, composite by Ruth Ashery-Padan (CC-BY 4.0).
Researchers have actually revealed brand-new insights into the causes of adult-onset macular degeneration.
A brand-new research study by Ran Elkon and Ruth Ashery-Padan of Tel Aviv University and colleagues has revealed a previously unidentified genetic danger aspect for adult-onset macular degeneration (AMD) by combining a map of gene regulative sites with disease-related loci. This discovery advances the understanding of the main cause of visual loss in grownups.
The findings were recently released in the journal PLOS Biology..
The leading reason for adult-onset macular degeneration (AMD) is due to a breakdown in the function of the retinal pigmented epithelium (RPE), a layer of tissue in between the photoreceptors that detect light and the choriocapillaris that offers nutrition to the retina. Recognizing the significance of RPE in the development of AMD, the authors of the study concentrated on checking out the function of a transcription factor called LHX2. The teams analysis of mouse mutants showed that LHX2 plays a vital role in RPE development.