The researchers revealed the molecular structure of a protein called uncoupling protein 1 (UCP1), responsible for enabling brown fat tissue to burn calories as heat, contrasting with standard white fat that shops calories.” As well as the conventional white fat that we are all familiar with, we can likewise establish brown fat. Brown fat is the great fat– it breaks down blood sugar and fat molecules to create heat and aid maintain body temperature level. Many of our fat, however, is white fat, which shops energy– and too much white fat leads to weight problems.” A lot of research study has been focusing on finding ways to motivate brown fat and how to turn white fat into brown fat– in order to burn more calories and combat metabolic illness.
Researchers have actually made a significant discovery in the understanding of weight problems and associated illness like diabetes. The scientists revealed the molecular structure of a protein called uncoupling protein 1 (UCP1), responsible for enabling brown fat tissue to burn calories as heat, contrasting with standard white fat that stores calories. This could result in the development of treatments that synthetically trigger UCP1 to burn off excess calories from fat and sugar, potentially combating obesity and diabetes.
Researchers have actually uncovered the molecular structure of the protein UCP1, which permits brown fat tissue to burn calories as heat. This breakthrough might pave the method for treatments that synthetically activate UCP1, consequently combating obesity and diabetes by burning off excess calories.
A St Catharines PhD trainee and among the Colleges alumni belong to a team of researchers at the University of Cambridge and the University of East Anglia who made a crucial discovery in the race to discover treatments for obesity and associated diseases, such as diabetes.
Scott Jones is completing his PhD at the University of Cambridges MRC Mitochondrial Biology Unit and is the first author of the new research study, which revealed for the very first time the molecular structure of a protein called uncoupling protein 1 (UCP1). This protein enables brown fat tissue, or good fat, to burn calories as heat– in contrast to conventional white fat which shops calories.
Scott stated, “Solving the structure of human uncoupling protein has been the focus of my PhD research studies so Im happy the structure is now published enabling us to further comprehend how the protein works and is managed.”
Alumnus Dr. Martin King (2002, Natural Sciences) is likewise based at the University of Cambridges MRC Mitochondrial Biology Unit and was a co-author of the paper published recently in the journal Science Advances. The advancement was made thanks to a global partnership between the UK group and coworkers at the University of Pennsylvania and the Free University of Brussels.
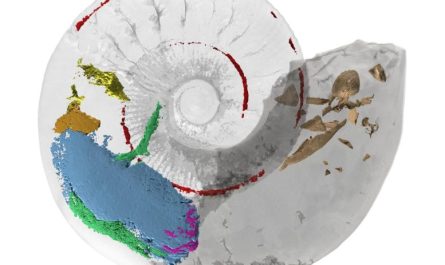
The human uncoupling protein in brown fat in its non-active form (left), hindered by a nucleotide, and in its activated form (right), which short-circuits the mitochondrion to produce heat. Credit: Penn Medicine
Dr. King explained, “This has actually been a long-lasting job (covering more than 10 years) and is a great example of how productive cooperations in science can be. Each lab has its own know-how that has been vital in solving this problem.”
These findings provide important molecular information that will assist develop therapeutics that trigger UCP1 synthetically to burn excess calories from fat and sugar. And that this could one day fight obesity and associated diseases, such as diabetes.
Dr. Paul Crichton from the University of East Anglia said:
” As well as the standard white fat that we are all familiar with, we can likewise establish brown fat. Brown fat is the excellent fat– it breaks down blood sugar and fat molecules to develop heat and aid maintain body temperature level. The majority of our fat, however, is white fat, which shops energy– and too much white fat causes obesity. UCP1 is the crucial protein that allows the specialized brown fat to burn off calories as heat.
” We know that mammals switch on UCP1 activity in brown fat tissue to secure versus the cold and to keep body temperature– specifically in new-borns, that can not yet shiver to keep warm. Brown fat varies in people, where it correlates with leanness in the population– and there has actually been a great deal of interest in how to increase brown fat and activate UCP1 therapeutically, as a prospective method to treat weight problems.
” A lot of research study has actually been focusing on finding methods to motivate brown fat and how to turn white fat into brown fat– in order to burn more calories and fight metabolic illness. Even with more brown fat– UCP1 needs to still be switched on to get full advantage.
Using the Krios G3i, a cryogenic electron microscopic lense at the Penn Singh Center for Nanotechnology, the group was able to see UCP1 in atomic detail.
Lead scientist from the University of Cambridge, Professor Edmund Kunji, stated:
” Our paper exposes, for the very first time, the structure of UCP1 in atomic information, and how its activity in brown fat cells is prevented by an essential regulatory molecule. Our work reveals how a regulator binds to prevent UCP1 activity, however more notably, the structure will enable scientists to rationalize how triggering particles bind to switch the protein on, leading to the burning of fat.
For more on this research, see The Breakthrough That Could Lead to New Obesity Treatments.
Reference: “Structural basis of purine nucleotide inhibition of human uncoupling protein 1” by Scott A. Jones, Prerana Gogoi, Jonathan J. Ruprecht, Martin S. King, Yang Lee, Thomas Zögg, Els Pardon, Deepak Chand, Stefan Steimle, Danielle M. Copeman, Camila A. Cotrim, Jan Steyaert, Paul G. Crichton, Vera Moiseenkova-Bell, Edmund R. S. Kunji, 31 May 2023, Science Advances.DOI: 10.1126/ sciadv.adh4251.
This research was supported by the Medical Research Council, the Biological and Biotechnological Sciences Research Council, and by the National Institutes of Health/National Institute of General Medical Sciences. Nanobody discovery was moneyed by the Instruct-ERIC part of the European Strategy Forum on Research infrastructures, and the Research Foundation– Flanders, and the Strategic Research Program of the Vrije Universiteit Brussel.