In a research study published today (December 27, 2021) in Developmental Cell, a group led by scientists at the University of Cambridge utilized genetically engineered mice to reveal how the fetus produces a signal to motivate development of blood vessels within the placenta. This signal likewise causes adjustments to other cells of the placenta to permit more nutrients from the mother to go through to the fetus.
Dr. Ionel Sandovici, the papers very first author, stated: “As it grows in the womb, the fetus requires food from its mum, and healthy capillary in the placenta are important to assist it get the appropriate amount of nutrients it needs.
” Weve recognized one manner in which the fetus utilizes to interact with the placenta to prompt the appropriate growth of these capillary. When this interaction breaks down, the blood vessels do not establish properly and the baby will struggle to get all the food it needs.”
The team discovered that the fetus sends a signal referred to as IGF2 that reaches the placenta through the umbilical cord. In humans, levels of IGF2 in the umbilical cable progressively increase in between 29 weeks of gestation and term: excessive IGF2 is related to too much development, while inadequate IGF2 is connected with insufficient development. Babies that are too large or too small are more most likely to suffer and even pass away at birth, and have a higher danger to establish diabetes and heart problems as adults.
Dr. Sandovici included: “Weve known for some time that IGF2 promotes the growth of the organs where it is produced. In this study, weve revealed that IGF2 also acts like a classical hormone– its produced by the fetus, goes into the fetal blood, through the umbilical cable and to the placenta, where it acts.”
Especially intriguing is what their findings reveal about the tussle occurring in the womb.
In mice, the action to IGF2 in the capillary of the placenta is mediated by another protein, called IGF2R. The 2 genes that produce IGF2 and IGF2R are inscribed– a process by which molecular switches on the genes identify their parental origin and can turn the genes on or off. In this case, just the copy of the igf2 gene inherited from the father is active, while just the copy of igf2r inherited from the mother is active.
Lead author Dr. Miguel Constância, stated: “One theory about imprinted genes is that paternally-expressed genes are greedy and self-centered. They wish to extract the most resources as possible from the mother. But maternally-expressed genes act as countermeasures to stabilize these demands.”
” In our study, the daddys gene drives the fetuss needs for larger blood vessels and more nutrients, while the mothers gene in the placenta attempts to control how much nutrition she offers. Theres a tug-of-war occurring, a battle of the sexes at the level of the genome.”
The group say their findings will allow a much better understanding of how the mom, placenta, and fetus communicate with each other during pregnancy. This in turn could cause methods of determining levels of IGF2 in the fetus and finding ways to utilize medication to stabilize these levels or promote regular advancement of placental vasculature.
The researchers utilized mice, as it is possible to manipulate their genes to simulate various developmental conditions. This enables them to study in information the various systems occurring. The physiology and biology of mice have numerous similarities with those of people, enabling researchers to design human pregnancy, in order to comprehend it better.
Recommendation: “The Imprinted Igf2-Igf2r Axis is Critical for Matching Placental Microvasculature Expansion to Fetal Growth” by Sandovici, I et al., 27 December 2021, Developmental Cell.DOI: 10.1016/ j.devcel.2021.12.005.
The lead researchers are based at the Department of Obstetrics and Gynaecology, the Medical Research Council Metabolic Diseases Unit, part of the Wellcome-MRC Institute of Metabolic Science, and the Centre for Trophoblast Research, all at the University of Cambridge.
The research study was largely funded by the Biotechnology and Biological Sciences Research Council, Medical Research Council, Wellcome Trust and Centre for Trophoblast Research.
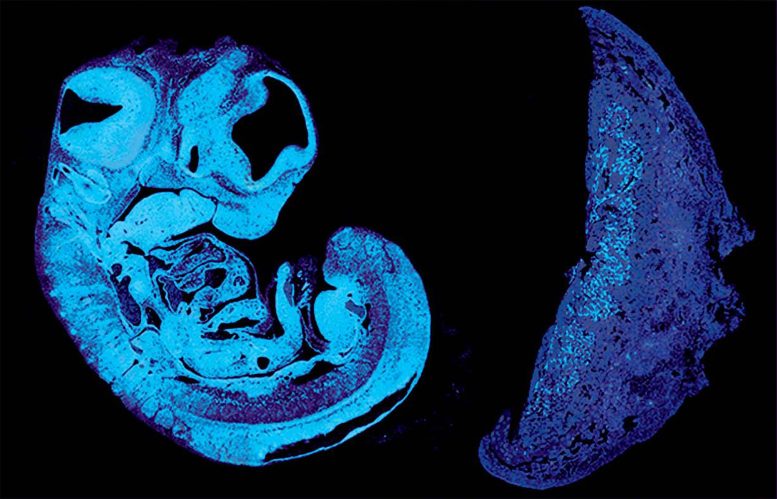
Areas of the fetus and placenta. Credit: Ionel Sandovici
Cambridge researchers have actually determined an essential signal that the fetus uses to manage its supply of nutrients from the placenta, revealing a tug-of-war in between genes acquired from the father and from the mom. The study, performed in mice, might assist describe why some babies grow badly in the womb.
As the fetus grows, it requires to communicate its increasing requirements for food to the mom. It gets its nutrition via blood vessels in the placenta, a specialized organ that consists of cells from both infant and mother.
In between 10% and 15% of children grow improperly in the womb, often revealing minimized growth of capillary in the placenta. In humans, these blood vessels expand significantly between mid and late pregnancy, reaching an overall length of around 320 kilometers at term.
The team discovered that the fetus sends out a signal understood as IGF2 that reaches the placenta through the umbilical cord. In mice, the action to IGF2 in the blood vessels of the placenta is mediated by another protein, called IGF2R. The two genes that produce IGF2 and IGF2R are imprinted– a procedure by which molecular switches on the genes identify their adult origin and can turn the genes on or off. In this case, just the copy of the igf2 gene acquired from the father is active, while just the copy of igf2r acquired from the mother is active.
Lead author Dr. Miguel Constância, stated: “One theory about imprinted genes is that paternally-expressed genes are greedy and selfish.