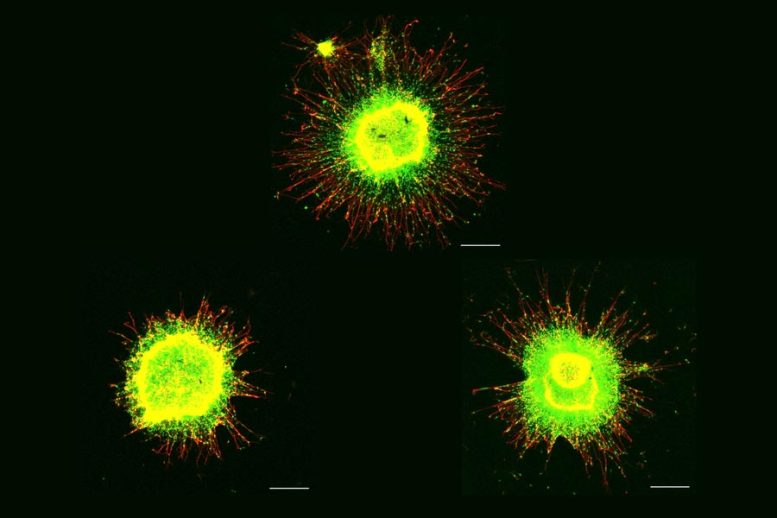
Neural progenitor cells with the typical number of chromosomes reveal substantial external migration in culture (top). The cells in the bottom left are untreated trisomy 21 cells.
Additional chromosome modifies chromosomal conformation and DNA availability in neural progenitor cells; research study establishes senescence as a possibly targetable system for future treatment.
In Down syndrome, the third copy of chromosome 21 causes a reorganization of the 3D configuration of the whole genome in a crucial cell kind of the developing brain, a brand-new study programs. The resulting interruption of gene transcription and cell function are so similar to those seen in cellular aging, or senescence, that the researchers leading the research study found they could use anti-senescence drugs to fix them in cell cultures.
The study released in Cell Stem Cell therefore develops senescence as a potentially targetable mechanism for future treatment of Down syndrome, states Hiruy Meharena, who led the work as a Senior Alana Fellow in the Alana Down Syndrome Center at MIT and is now an assistant professor at the University of California at San Diego.
Neural progenitor cells with the normal number of chromosomes show substantial outward migration in culture (top). The cells in the bottom left are without treatment trisomy 21 cells. Meharena and co-authors spent years determining differences between human cell cultures that varied just by whether they had a third copy of chromosome 21. In both the stem cells and the NPCs, the team analyzed 3D chromosome architecture, several metrics of DNA structure and interaction, gene ease of access and transcription, and gene expression. These changes and differences in DNA conformation within the cell nucleus lead to modifications in how genes are transcribed and therefore expressed, triggering crucial differences in cell function that impact brain development.
” There is a cell-type-specific genome-wide disruption that is independent of the gene dose reaction,” Meharena states. “Its a really similar phenomenon to whats observed in senescence. This suggests that extreme senescence in the developing brain caused by the third copy of chromosome 21 could be a crucial reason for the neurodevelopmental abnormalities seen in Down syndrome.”
Li-Huei Tsai and Hiruy Meharena speak with about images produced during the research study in this 2019 picture. Credit: David Orenstein/The Picower Institute
The studys finding that neural progenitor cells (NPCs), which establish into significant cells in the brain consisting of nerve cells, have a senescent character is amazing and unique, states senior author Li-Huei Tsai, but it is substantiated by the teams substantial work to illuminate the underlying system of the impacts of abnormal chromosome number, or aneupoloidy, within the nucleus of the cells.
” This research study shows the significance of asking basic concerns about the hidden systems of neurological conditions,” says Tsai, Picower Professor of Neuroscience, director of the Alana Center, and of the Picower Institute for Learning and Memory at MIT. “We didnt begin this work anticipating to see senescence as a translationally appropriate feature of Down syndrome, but the information emerged from asking how the existence of an extra chromosome impacts the architecture of all of a cells chromosomes during advancement.”
Genome-wide modifications
Meharena and co-authors spent years determining differences between human cell cultures that varied only by whether they had a 3rd copy of chromosome 21. Stem cells originated from volunteers were cultured to turn into NPCs. In both the stem cells and the NPCs, the team examined 3D chromosome architecture, numerous metrics of DNA structure and interaction, gene ease of access and transcription, and gene expression. They likewise looked at the repercussions of the gene expression differences on important functions of these developmental cells, such as how well they moved and multiplied in 3D brain tissue cultures. Stem cells were not especially different, but NPCs were considerably impacted by the 3rd copy of chromosome 21.
Overall, the photo that emerged in NPCs was that the presence of a 3rd copy triggers all the other chromosomes to crush inward, not unlike when individuals in a crowded elevator should narrow their position when another person squeezes in. The main results of this “chromosomal introversion,” thoroughly quantified in the research study, are more hereditary interactions within each chromosome and fewer interactions amongst them. These modifications and differences in DNA conformation within the cell nucleus result in modifications in how genes are transcribed and for that reason revealed, causing essential distinctions in cell function that impact brain advancement.
Dealt with as senescence
For the first number of years as these data emerged, Meharena says, the complete significance of the genomic changes were not evident, however then he checked out a paper revealing extremely similar genomic rearrangement and transcriptional modifications in senescent cells.
After validating that the Down syndrome cells indeed bore such a comparable signature of transcriptional distinctions, the group chose to test whether anti-senecence drugs could undo the impacts. They evaluated a combination of 2: dasatinib and quercetin. The medications enhanced not just gene accessibility and transcription, however likewise the migration and expansion of cells.
That said, the drugs have really significant adverse effects– dasatinib is just provided to cancer patients when other treatments have actually not done enough– so they are not appropriate for attempting to intervene in brain development amid Down syndrome, Meharena says. Rather, a result of the research study could be to motivate a look for medications that might have anti-senolytic effects with a much safer profile.
Senescence is a stress response of cells. At the very same time, years of research study by the late MIT teacher of biology Angelika Amon, who co-directed the Alana Center with Tsai, has actually revealed that aneuploidy provides considerable tension for cells. A question raised by the new findings, for that reason, is whether the senescence-like character of Down syndrome NPCs is certainly the result of an aneuploidy-induced tension and, if so, exactly what that stress is.
Another implication of the findings is how extreme senescence amongst brain cells might affect individuals with Down syndrome later on in life. The threat of Alzheimers illness is much higher at a substantially earlier age in the Down syndrome population than amongst individuals in general. In big part this is thought to be due to the fact that an essential Alzheimers risk gene, APP, is on chromosome 21, but the freshly determined disposition for senescence may also speed up Alzheimers development.
Recommendation: “Down-syndrome-induced senescence interrupts the nuclear architecture of neural progenitors” by Hiruy S. Meharena, Asaf Marco, Vishnu Dileep, Elana R. Lockshin, Grace Y. Akatsu, James Mullahoo, L. Ashley Watson, Tak Ko, Lindsey N. Guerin, Fatema Abdurrob, Shruthi Rengarajan, Malvina Papanastasiou, Jacob D. Jaffe and Li-Huei Tsai, 6 January 2022, Cell Stem Cell.DOI: 10.1016/ j.stem.2021.12.002.
In addition to Meharena and Tsai, the papers other authors are Asaf Marco, Vishnu Dileep, Elana Lockshin, Grace Akatsu, James Mullahoo, Ashley Watson, Tak Ko, Lindsey Guerin, Fatema Abdurrob, Shruti Rengarajan, Malvina Papanastasiou and Jacob Jaffe.
The Alana Foundation, the LuMind Foundation, Burroughs Wellcome Fund, UNCF-Merck, and the National Institutes of Health moneyed the research.