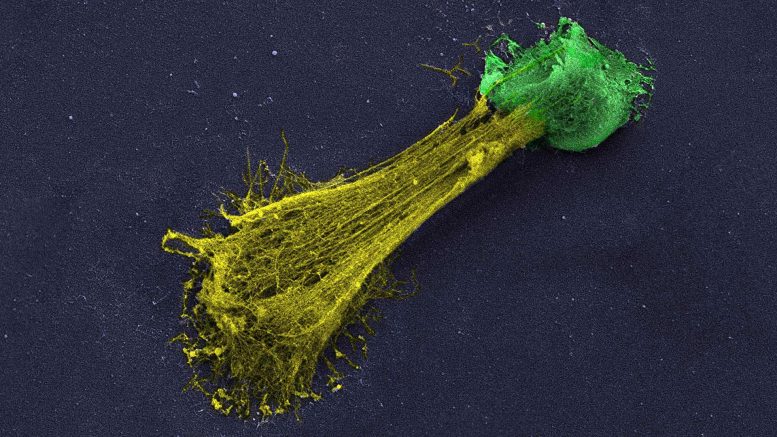
A high resolution microscopic image of a neutrophil extracellular trap (NET). Neutrophils (colored green) are white blood cells that help the immune system battle off invaders. These nets or networks can hire additional neutrophils. Jose M. Adrover, a postdoctoral fellow in CSHL Professor Mikala Egeblads laboratory, explains that NETs are generally launched throughout infections when immune cells, called neutrophils, challenge a threat that is too big for the small cells to fight straight. They discovered that the drug avoids neutrophils isolated from blood from generating NETs.
A high resolution microscopic picture of a neutrophil extracellular trap (NET). Neutrophils (colored green) are white blood cells that assist the body immune system eradicate intruders. Neutrophils can commit suicide and spill out DNA and chemical signals into a sticky network of filaments (colored yellow). These nets or networks can recruit extra neutrophils. Having a lot of neutrophils can lead to tissue damage. Credit: Jose M. Adrover/Egeblad lab/CSHL, 2022
A team of researchers led by Cold Spring Harbor Laboratory (CSHL) have found that disulfiram, an FDA-approved drug, prevents the immune system from producing harmful webs called neutrophil extracellular traps (NETs). Many scientists believe NETs assist drive the development of severe breathing distress syndrome (ARDS) in patients with extreme COVID-19 and other dangerous lung injuries.
Jose M. Adrover, a postdoctoral fellow in CSHL Professor Mikala Egeblads lab, discusses that NETs are usually launched throughout infections when immune cells, called neutrophils, confront a threat that is too large for the tiny cells to fight directly. To extend their reach, neutrophils spew a sticky web of DNA and toxins, which indiscriminately toxins pathogens and the bodys own cells. “They will harm whatever, all around,” Adrover states.
Since NETs can be so harmful, scientists in Egeblads laboratory have actually been looking for methods to obstruct their development. Disulfiram, which has actually been used considering that the 1950s as a treatment for alcohol usage disorders, was an appealing candidate. “Disulfiram disrupts gasdermin D, a molecule required to produce NETs”, says Juliane Daßler-Plenker, a postdoctoral fellow in Egeblads laboratory.
The team that consisted of Weill Cornell Medicine (WCM), and Icahn School of Medicine at Mount Sinai (Mt. Sinai) examined disulfirams impacts on NET production. They found that the drug avoids neutrophils isolated from blood from producing NETs. Then, they gave disulfiram to mice with intense lung injuries: “By digital tomography [ CT scan], we saw a plain decrease of edema [fluid] in the lungs, and the drug considerably improved survival,” states Scott Lyons, head of Animal Imaging at CSHL. Robert Schwartzs team (WCM) and Benjamin tenOevers group (Mt. Sinai) checked disulfiram in hamsters infected with the SARS-CoV-2 infection; NET production was obstructed and lung injury was minimized.
Disulfiram is the first FDA-approved drug that can obstruct NET formation. In this research study, Egeblads group dissects the drugs capability to block NETs and change immune signaling in a way that might be advantageous for dealing with severe COVID-19. Their results are reported in JCI Insight.
Clinical trials examining disulfirams usage in patients with symptomatic COVID-19 are underway, and while CSHL scientists are not included in those studies, Egeblad says, “Our findings supply a reason to hope that disulfiram may be an useful treatment. We will continue exploring the drugs capacity in a range of conditions. Just as essential, we now have a tool to assist us study the complex functions of NETs in lung injury, cancer, and other illness that involve NETs.”
Reference: “Disulfiram prevents neutrophil extracellular trap development securing rodents from severe lung injury and SARS-CoV-2 infection” 8 February 2022, JCI Insight.DOI: 10.1172/ jci.insight.157342.
Financing: Dr. Marcia Kramer Mayer, William C. and Joyce C. ONeil Charitable Trust, CSHL Cancer Center, NIH/National Institute of General Medical Sciences, NIH/National Cancer Institute, NIH/National Institute of Diabetes and Digestive and Kidney Diseases, Department of Medicine, Weill Cornell Medicine, Irma Hirschl Trust Research Award.