In mammals, oocytes establish from germ cells, precursor cells that move from early embryonic tissue to the developing gonads. Bacterium cells then go through meiosis, an important chromosomal rearrangement process, which is responsible for the hereditary uniqueness of each individual bacterium cell.
To resolve this question, scientists at the Centre for Genomic Regulation (CRG) constructed an X-chromosome reporter system (XRep), a tool that permitted them to study how the chromosome shapeshifts with time throughout germ cell development in vitro.
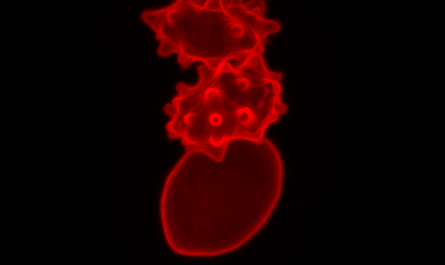
Image showing synthetic ovary (left), where in vitro-generated female germ cells (magenta) enter meiosis (yellow cells) to end up being oocytes with the assistance of supporting cells (cyan). The chromosomal organization throughout the meiosis process is shown (lower right) when germ cells become distinct by reshuffling their hereditary product.
Utilizing female mouse cells, the method revealed a carefully orchestrated act of X-chromosome yoyo. If one X chromosome is briefly suspended and after that reactivated, it resulted in germ cells being four times more effective for getting in meiosis and changing into egg cells compared to germ cells that have actually never ever turned their X chromosome on and off again. In contrast, germ cells that stopped working to inactivate the X chromosome in the very first place or reactivated it too quickly showed abnormal gene expression and cell differentiation patterns.
The research study likewise discovered that cells with 2 active X chromosomes divided quicker and easily reverted into a state of pluripotency. According to the authors, these qualities are comparable to human germ cell tumors, which come type germ cells that got lost throughout their migration to the gonads or failed to differentiate properly once inside the testicles and ovaries. The researchers deduced that a right X-chromosome inactivation and reactivation sequence is a sign of regular bacterium cell differentiation. The research group keeps in mind that more studies will be required to validate whether an unusual X-chromosome state is a diagnostic indicator, or whether it could be a causal factor responsible for the cell problem.
” Our findings have important ramifications for reproductive research study since XRep allows us to examine cellular X-chromosome status in genuine time, assisting identify and separate germ cells with a high success rate of turning into oocytes,” states Dr. Bernhard Payer, Group Leader at the CRG and senior author of the study.
” Human oocytes have never ever been produced completely in vitro. Monitoring the X-chromosome state throughout in vitro germ cell distinction may for that reason be a way to optimize the procedure to produce premium human eggs in the laboratory. Since they are presently just readily available from egg donors and are primarily used for reproductive functions, human eggs for research are tough and scarce to get. In vitro-generated human eggs could for that reason supply an endless resource to study the causes and treatments of infertility conditions and also to evaluate the safety of drugs and chemicals for ladiess recreation,” concludes Dr. Payer.
Dr. Moritz Bauer, co-first author of the study, includes: “Our outcomes also highlight how we require specific tools to study female cells. The vast majority of studies are carried out using male cells, causing a gender-gap in scientific understanding. We therefore need to stop taking a look at female development through the lens of male cells, which will add to our understanding of sex-specific disease developments.”
Referral: “Controlled X-chromosome activation characteristics defines meiotic capacity of female mouse in vitro bacterium cells” 23 May 2022, The EMBO Journal.DOI: 10.15252/ embj.2021109457.
Image revealing synthetic ovary, where in vitro-generated female bacterium cells (magenta) go into meiosis (yellow cells) to become oocytes with aid from supporting cells (cyan). When the X chromosome is properly turned OFF and ON once again throughout germ cell development, this process is 4-times more effective. Credit: Dr. Jacqueline Severino/CRG
Discovery of yoyo mechanism might pave the method for producing artificial oocytes in the lab.
A prospective brand-new diagnostic marker that predicts the efficient and successful development of mammalian egg cells has actually been determined by researchers at the Center for Genomic Regulation (CRG) in Barcelona. The discovery might lead the way for producing artificial oocytes in the lab, assisting scientists study the causes and treatments of infertility conditions and test the impact of drugs and chemicals on femaless reproduction. The research study will be released today (May 23, 2022) in The EMBO Journal.
Humans have 23 pairs of chromosomes. In between males and females, 22 sets are shared, with the 23rd pair being the sex chromosomes. Males have an X and a Y chromosome, while females have 2 Xs. This triggers a prospective issue for the female cellular machinery, as 2 active X chromosomes generates an overdose of gene items, which is fatal for establishing embryos or results in cancer in adult life. To prevent this situation, female cells inactivate one X chromosome by shutting off its genes and condensing it.
Image revealing artificial ovary, where in vitro-generated female germ cells (magenta) go into meiosis (yellow cells) to end up being oocytes with assistance from supporting cells (cyan). Germ cells then go through meiosis, an important chromosomal rearrangement procedure, which is accountable for the genetic originality of each private germ cell. Image revealing artificial ovary (left), where in vitro-generated female bacterium cells (magenta) enter meiosis (yellow cells) to become oocytes with the aid of supporting cells (cyan). If one X chromosome is quickly suspended and then reactivated, it resulted in bacterium cells being four times more effective for getting in meiosis and transforming into egg cells compared to germ cells that have never ever turned their X chromosome off and on once again. According to the authors, these characteristics are comparable to human bacterium cell growths, which come type bacterium cells that got lost throughout their migration to the gonads or failed to separate properly once inside the testicles and ovaries.