The research study was little– lung samples from 18 cases and plasma samples from 6 of those cases– the researchers state their data exposed patterns that could assist establish brand-new COVID-19 rehabs and fine-tune when to use existing rehabs at various stages of disease development. The findings consist of information about how SARS-CoV-2, the virus that triggers COVID-19, spreads in the lungs, controls the immune system, triggers prevalent apoplexy that does not resolve, and targets signaling pathways that promote lung failure, fibrosis and hinder tissue repair work. Every case showed findings constant with diffuse alveolar damage, which prevents proper oxygen flow to the blood and ultimately makes lungs thickened and stiff.
They also found that SARS-CoV-2 directly infected basal epithelial cells within the lungs, hampering their essential function of repairing harmed respiratory tracts and lungs and generating healthy tissue. The process is different from the method influenza infections attack cells in the lungs. When evaluating or developing antiviral therapies, this supplies researchers with additional info to utilize.
Researchers at NIHs National Institute of Allergy and Infectious Diseases led the task in partnership with the National Institute of Biomedical Imaging and Bioengineering and the U.S. Food and Drug Administration. Other collaborators included the Institute for Systems Biology in Seattle; University of Illinois, Champaign; Saint Johns Cancer Institute in Santa Monica, California.; the USC Keck School of Medicine in Los Angeles; University of Washington Harborview Medical Center, Seattle; University of Vermont Medical Center, Burlington; and Memorial Sloan Kettering Cancer Center in New York City.
Reference: “Lung endothelial and epithelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in deadly COVID-19” by Felice DAgnillo, Kathie-Anne Walters, Yongli Xiao, Zong-Mei Sheng, Kelsey Scherler, Jaekeun Park, Sebastian Gygli, Luz Angela Rosas, Kaitlyn Sadtler, Heather Kalish, Charles A. Blatti, Ruoqing Zhu, Lisa Gatzke, Colleen Bushell, Matthew J. Memoli, Steven J. ODay, Trevan D. Fischer, Terese C. Hammond, Raymond C. Lee, J. Christian Cash, Matthew E. Powers, Grant E. OKeefe, Kelly J. Butnor, Amy V. Rapkiewicz, William D. Travis, Scott P. Layne, John C. Kash and Jeffery K. Taubenberger, 14 October 2021, Science Translational Medicine.DOI: 10.1126/ scitranslmed.abj7790.
Jeffrey Taubenberger, M.D., Ph.D., Chief of the Viral Pathogenesis and Evolution Section in NIAIDs Laboratory of Infectious Diseases, is offered to discuss this study.
They likewise discovered that SARS-CoV-2 directly contaminated basal epithelial cells within the lungs, restraining their vital function of fixing damaged airways and lungs and creating healthy tissue.
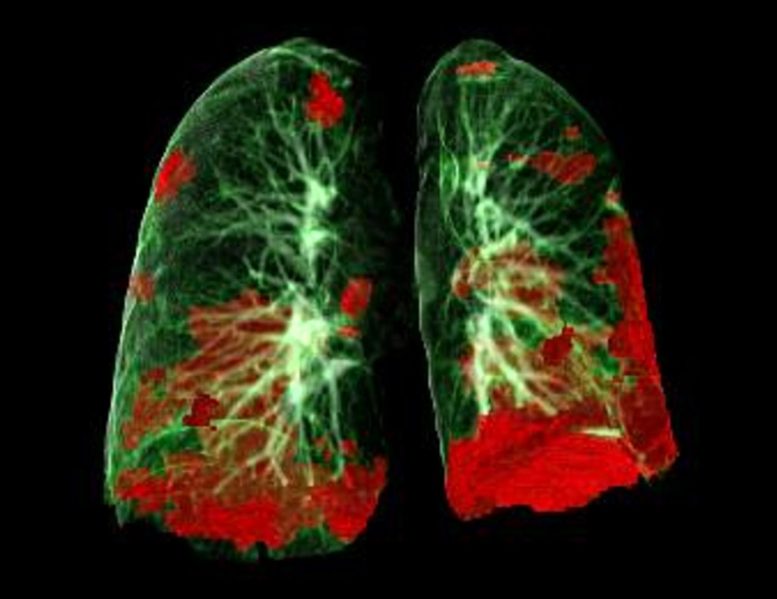
CT scan of patients lungs showing COVID-19 damage in red. Credit: Gerlig Widmann and group, Department of Radiology, Medical University of Innsbruck
SARS-CoV-2 avoids lung tissue repair, regrowth.
Lung autopsy and plasma samples from individuals who passed away of COVID-19 have supplied a clearer photo of how the SARS-CoV-2 infection spreads and damages lung tissue. Researchers at the National Institutes of Health and their collaborators say the details, published in Science Translational Medicine, could assist forecast extended and severe COVID-19 cases, especially among high-risk individuals, and notify effective treatments.
The research study was small– lung samples from 18 cases and plasma samples from 6 of those cases– the researchers state their data exposed patterns that could help develop new COVID-19 therapeutics and tweak when to use existing therapies at different phases of disease development. The findings consist of details about how SARS-CoV-2, the infection that causes COVID-19, spreads in the lungs, controls the immune system, triggers prevalent thrombosis that does not deal with, and targets signaling paths that promote lung failure, fibrosis and hinder tissue repair work. The researchers say the information are especially relevant to looking after COVID-19 patients who are elderly, obese, or have diabetes– all considered high-risk populations for extreme cases. Research study samples were from patients who had at least one high-risk condition.
Colorized scanning electron micrograph of a cell infected with an alternative stress of SARS-CoV-2 virus particles (blue), separated from a client sample. Image recorded at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
The study consisted of patients who passed away between March and July 2020, with time of death ranging from 3 to 47 days after signs began. This varied timeframe permitted the researchers to compare short, intermediate, and long-term cases. Every case revealed findings consistent with diffuse alveolar damage, which prevents appropriate oxygen circulation to the blood and ultimately makes lungs thickened and stiff.