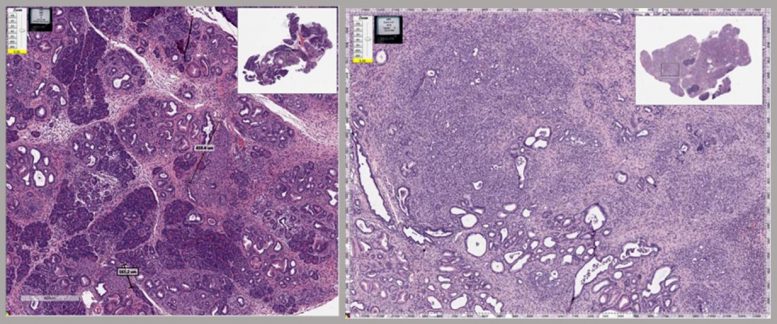
The image on the left (Control) is a magnified view of an example mouse pancreas that has actually developed pancreatic cancer due to mutations in cancer susceptibility genes and an inflammatory representative. On the right (MBZ) is the very same mouse strain treated with the same inflammatory representative, but mebendazole was included to the very same mouse feed, and it has little or no tiny proof of cancer or pathology. The study reveals that mebendazole may act likewise in pancreatic cancer by collapsing cancer cells structure, along with other mechanisms such as reducing swelling.
” We are promoting for use of mebendazole as a treatment for those identified prior to metastasis to see if we can slow or prevent pancreatic cancer,” Riggins says.
Riggins and his group administered mebendazole to mice that were genetically engineered to establish pancreatic cancer. The group measured the inflammation and the modification in tissue, along with the phase, grade and metastatic status in each tumor.
Originally used to battle roundworm, hookworm and other parasitic infections by cutting off the parasites supply of nutrition, mebendazole prevents the formation of tubulin. Tubulin, Riggins describes, is both a micro-skeleton of the inner cell and a highway for transportation. The drug enters the parasites gut and collapses the tubulin, starving the parasite to death. The study shows that mebendazole may act similarly in pancreatic cancer by collapsing cancer cells structure, in addition to other systems such as minimizing swelling.
Riggins says he wants to continue his teams research through human scientific trials.
” We are advocating for use of mebendazole as a therapy for those detected prior to transition to see if we can slow or avoid pancreatic cancer,” Riggins states. “For those with advanced cancers, it could be an alternative to particular surgical treatments. Mebendazole may have energy as a therapy after preliminary treatment to prevent growth reoccurrence in the 15% to 20% of pancreatic adenocarcinoma clients who undergo surgery. It may also increase the resilience of response to basic chemotherapy in the staying 80% to 85% of clients with innovative disease.”
Reference: “Mebendazole disrupts stromal desmoplasia and tumorigenesis in 2 designs of pancreatic cancer” by Tara Williamson, Michelle Carvalho de Abreu, Dimitri G. Trembath, Cory Brayton, Byunghak Kang, Thais Biude Mendes, Paulo Pimentel de Assumpção, Janete M. Cerutti and Gregory J. Riggins, 6 July 2021, Oncotarget.DOI: 10.18632/ oncotarget.28014.
The Virginia and D.K. Ludwig Fund for Cancer Research offered financing for the research study..
Other researchers who carried out the research include Tara Williamson, Michelle Carvalho de Abreu, Dimitri G. Trembath, Cory Brayton, Byunghak Kang, Thais Biude Mendes, Paulo Pimentel de Assumpção and Janete M. Cerutti.
Riggins and Williamson are innovators on intellectual property related to mebendazole owned and handled by Johns Hopkins University conflict of interest policies. Riggins has a monetary interest in Benizole Therapeutics, PBC..
The image left wing (Control) is an amplified view of an example mouse pancreas that has actually developed pancreatic cancer due to mutations in cancer vulnerability genes and an inflammatory agent. On the right (MBZ) is the very same mouse stress treated with the same inflammatory representative, but mebendazole was added to the exact same mouse feed, and it has little or no tiny proof of cancer or pathology. Credit: Tara Williamson
As the third-most lethal cancer in the United States, with only a 1% five-year survival rate for individuals with its most aggressive kind, pancreatic cancer has long been a target of scientists who look for ways to slow or stop its development and spread. Now, a team of Johns Hopkins Medicine researchers have actually found that an anti-parasitic drug prevents pancreatic cancers initiation, progression, and metastasis in genetically engineered mice.
In a study released in the journal Oncotarget, Gregory Riggins, M.D., Ph.D., professor of neurosurgery and oncology at the Johns Hopkins University School of Medicine, and his team utilized 2 various mouse models to determine that the anti-parasitic drug mebendazole might slow or stop the development and spread of both early and late-stage pancreatic cancer.
” We believe that mebendazole might have a role in all phases,” Riggins says. “It was especially efficient for pancreatic cancer that was spotted early.”