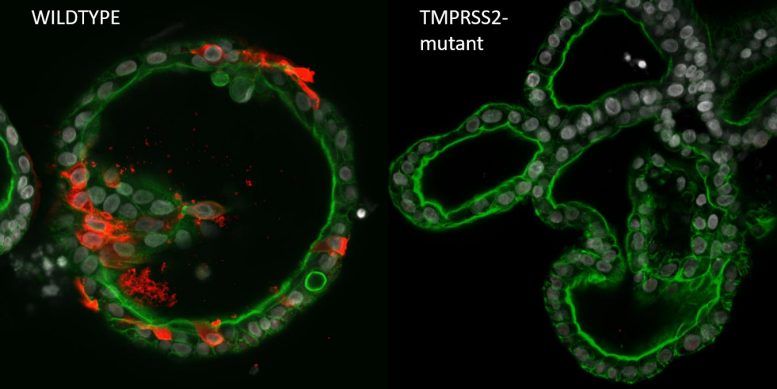
SARS-CoV-2 (shown by nucleocapsid, red) effectively infects wildtype human digestive organoids (left), while TMPRSS2-deficient organoids are completely lacking virus (right). Nuclei are grey, phalloidin (green) visualizes actin filaments. Credit: Joep Beumer, copyright Hubrecht Institute
Scientists from the group of Hans Clevers in cooperation with the group of Bart Haagmans (Erasmus MC) established an organoid biobank to look for the genes that are vital for the spreading of a SARS-CoV2 infection. Their study was released in Nature Communications on September 17, 2021, and highlights the usefulness of organoids for basic research into coronaviruses, along with highlighting possible drug targets.
Organoids are tiny 3D structures grown from stem cells that simulate organ function. Scientists have actually formerly succeeded in developing organoid models for various organs, including the gut, lung, uterus, and even the snake venom gland. The organoids have actually shown useful for diagnostic functions, forecasting treatment reactions in clients, and opening tricks about the advancement of tissues and rare cell types.
Host factors
Researchers can include levels of intricacy to the organoid cultures for specific functions. They can for example include immune cells to tumor organoids to study the efficacy of therapy, or inject pathogens into organoids to design their result on the cells.
SARS-CoV-2 (indicated by nucleocapsid, red) effectively infects wildtype human digestive organoids (left), while TMPRSS2-deficient organoids are totally devoid of infection (right). The organoids have actually shown beneficial for diagnostic purposes, anticipating treatment actions in patients, and unlocking secrets about the development of tissues and rare cell types.
They can for example add immune cells to tumor organoids to study the effectiveness of therapy, or inject pathogens into organoids to model their effect on the cells. When duplicating the Chloroquine-experiments using the mutant organoids instead of previously used cell lines, the research study groups observed no restorative impact. In other words, the outcomes in organoids were comparable to the results in medical trials, suggesting that– compared to animal cell lines– the organoids could be much better fit to predict the effectiveness of therapeutics in humans.
Biobank of digestive organoids.
To discover more about the host aspects that are particularly crucial for the duplication and dispersing of coronaviruses, the groups of Hans Clevers and Bart Haagmans established a biobank of mutant digestive organoids. This implies that the organoids, that imitate the biology of the intestine, consisted of numerous anomalies in the host aspects that were previously discovered to be appropriate for coronaviruses. These anomalies cause changes in the activity of the host elements.
TMPRSS2 as restorative target.
The scientists injected the mutant organoids with SARS-CoV-2– the virus responsible for COVID-19– to study the impact of the mutations on the duplication and dispersing of the virus. Among other things, they identified the gene TMPRSS2 to be involved in this procedure: organoids with non-functioning TMPRSS2 revealed reduced duplication and spreading of the infection.
Significance of human models.
Previously, studies used cell lines of animals (most importantly, of the African green monkey) to identify therapeutic targets for the coronavirus. These cell lines are easy to work with, but do not fully recapitulate the biology of human cells that are targets of SARS-CoV-2. This is shown by the anti-malaria drug Chloroquine, which was found to be reliable versus SARS-CoV-2 infection in these cell lines, however turned out to be inefficient in medical trials with patients. This indicates that the cell lines can not sufficiently forecast the efficiency of therapies in human beings. When repeating the Chloroquine-experiments using the mutant organoids instead of formerly used cell lines, the research groups observed no therapeutic result. In other words, the lead to organoids were comparable to the outcomes in clinical trials, recommending that– compared to animal cell lines– the organoids could be much better matched to predict the efficiency of therapeutics in humans.
Future infections.
With their research study, published in Nature Communications, the groups of Hans Clevers and Bart Haagmans highlight the importance of organoids for research study into coronaviruses. They determine TMPSS2 as a possible restorative target for SARS-CoV2. Their recently established biobank could also aid in screening future emerging viruses to rapidly identify healing targets.
Recommendation: “A CRISPR/Cas9 genetically engineered organoid biobank reveals essential host aspects for coronaviruses” by Joep Beumer, Maarten H. Geurts, Mart M. Lamers, Jens Puschhof, Jingshu Zhang, Jelte van der Vaart, Anna Z. Mykytyn, Tim I. Breugem, Samra Riesebosch, Debby Schipper, Petra B. van den Doel, Wim de Lau, Cayetano Pleguezuelos-Manzano, Georg Busslinger, Bart L. Haagmans and Hans Clevers, 17 September 2021, Nature Communications ( 2021 ).
Hans Clevers is primary private investigator at the Hubrecht Institute and the Princess Máxima Center for Pediatric Oncology, professor of Molecular Genetics at the UMC Utrecht and Utrecht University, and Oncode Investigator.
Bart Haagmans is a primary detective at the Viroscience department at the Erasmus MC University Medical Center Rotterdam.