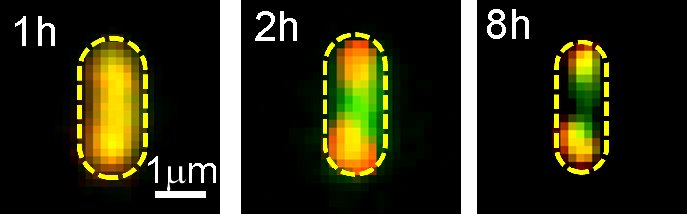
In the teams newest study, they examined the dynamics of aggresome formation as well as their function.Using fluorescently labeled proteins that the team had actually previously shown accumulate in aggresomes, the scientists discovered that upon ATP deficiency, the aggresomes form slowly over a number of hours, normally resulting in one at each end of the rod-shaped bacteria. With a special type of fluorescence microscopy called fast super-resolved single-molecule tracking, which follows the motions of individual labeled proteins, they likewise showed that within the aggresomes the proteins freely diffuse in a method that suggests theyre moving through a liquid environment.Aggresome formation, as revealed by fluorescently identified proteins (red and green), in an E. coli cellCOURTESY OF MARK LEAKE, UNIVERSITY OF YORKFurther evidence that the aggresomes are liquid came from experiments revealing they could be liquified by an organic solvent that can not dissolve solids and that fluorescently identified proteins diffuse from one half of an aggresome to the other. In addition, computer simulations revealed, among other things, that rather than being an active procedure, no input of energy was required for aggresome development– that is, the globs of proteins establish “at thermal balance,” says Leake, which is a sign of real LLPS.Although the group does not yet know how the aggresomes form, they presume it might have something to do with ATP itself.
In the teams most current study, they examined the dynamics of aggresome development as well as their function.Using fluorescently identified proteins that the team had actually formerly revealed build up in aggresomes, the researchers found that upon ATP exhaustion, the aggresomes form gradually over a number of hours, generally resulting in one at each end of the rod-shaped germs. With a special type of fluorescence microscopy called fast super-resolved single-molecule tracking, which follows the motions of individual labeled proteins, they also revealed that within the aggresomes the proteins easily diffuse in a method that suggests theyre moving through a liquid environment.Aggresome development, as shown by fluorescently labelled proteins (red and green), in an E. coli cellCOURTESY OF MARK LEAKE, UNIVERSITY OF YORKFurther evidence that the aggresomes are liquid came from experiments showing they could be liquified by an organic solvent that can not liquify solids and that fluorescently identified proteins diffuse from one half of an aggresome to the other. Furthermore, computer system simulations showed, among other things, that rather than being an active process, no input of energy was needed for aggresome development– that is, the globs of proteins develop “at thermal balance,” says Leake, which is a sign of real LLPS.Although the team does not yet understand how the aggresomes form, they believe it might have something to do with ATP itself. Thus, its depletion may stimulate hydrophobic proteins to gather together.Regardless of the system, “the major finding of our paper is to show that in aggresomes proteins tend to remain together but not strictly together.