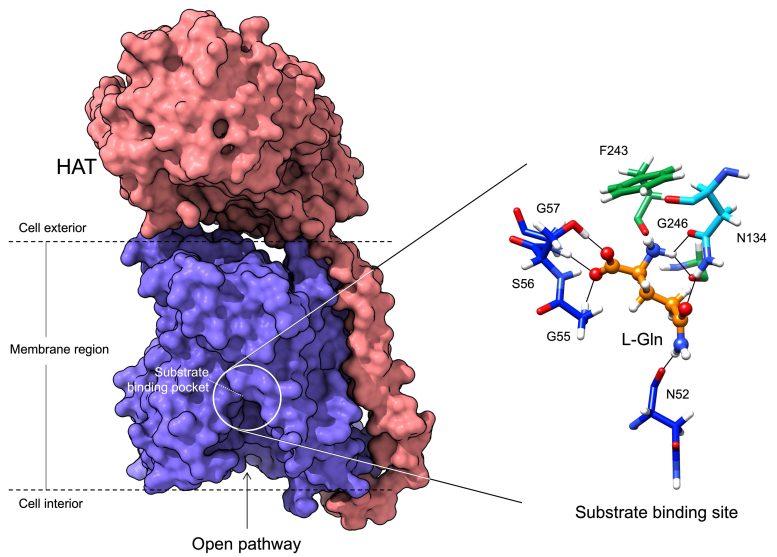
In blue, the region that binds the amino acids transported in and out of the cells. The amino acid binding center of this region is highlighted. Molecular characteristics and modeling were utilized to determine how different amino acids bind the transporter. The proteins that belong to the HAT household are necessary for life as they transport amino acids across the cell membrane. The members of this family are virtually similar, some transport certain amino acids and not others.
In blue, the region that binds the amino acids transferred in and out of the cells. The amino acid binding center of this region is highlighted. Molecular dynamics and modeling were utilized to figure out how various amino acids bind the transporter. Credit: Oscar Llorca (CNIO).
The proteins that belong to the HAT family are vital for life as they transfer amino acids across the cell membrane. The members of this household are almost similar, some transport certain amino acids and not others.
Thanks to the most recent high-resolution structural technologies such as cryo-electron microscopy, integrated with computational modeling and the design of mutants of these proteins, the scientists have had the ability to observe the structure of one of the members of this protein family in atomic detail and decipher its function. The results of the research study reveal how only a couple of residues– located in defined areas– of this household of proteins pick the particular amino acids to which they will bind and are therefore responsible for the protein to take part in specific physiological functions.
Equipped with this details, the scientists now face the difficulty of finding brand-new therapies and diagnostic tools for diseases that involve the HAT family of transporter proteins, with a specific interest in those conditions that pose severe illness, such as cancer and neurodegenerative conditions such as Alzheimer ´ s illness. Comprehending how to interrupt their function.
Amino acids, the basic foundation of life, go into and leave cells, allowing them to grow, divide and establish their functions. This movement into and out of the cell takes place thanks to gates embedded in the cell membrane that are formed by proteins of the HAT household, to name a few.
Although HAT proteins are practically identical in structure, some transport particular amino acids and not others, thus giving each member of the family particular functions, such as the participation in cell development; a function in diseases such as cancer; neuronal functions; and the transportation of harmful substances and participation in dependency to compounds such as drug.
To comprehend this specificity of function, the scientists set out to study the 3D structure of this crucial family of proteins. “Classical methods used to determine the structure of proteins, such as those utilizing X-rays, have had restricted success with proteins that are embedded in biological membranes, and so many questions have remained unresolved,” states Oscar Llorca, head of the Macromolecular Complexes in DNA Damage Response Group at CNIO, Director of the Structural Biology Programme of this Centre and co-author of the work.
” The combination of structural resolution by cryo-electron microscopy with molecular characteristics estimations and practical research studies supplies a speculative platform with a lot of potential that allows us to unravel the function of amino acid transporters. In this case, we have used this innovation to recognize the molecular mechanisms that lead these proteins to transfer some amino acids however not others,” states Manuel Palacín, head of the Amino Acid and Disease Transporters laboratory at IRB Barcelona, teacher of the University of Barcelona and Unit leader of CIBERER.
New drugs versus cancer and Alzheimer ´ s disease.
Thanks to cryo-electron microscopy, a field in which Llorca is a worldwide professional, visualization of the molecular structure of proteins has actually taken a giant step towards what we now called the golden age of 3D structures. This new innovation, which won the Nobel Prize in Chemistry in 2017, has not only served to observe biological procedures like never ever before however it is likewise adding to accelerating the advancement of new substances and drugs of interest to treat cancer and other human illness.
In this work, utilizing cryo-electron microscopy, the scientists have been able to imagine the structure of a member of the HAT family at atomic resolution and identify the pocket where these proteins bind to amino acids, in addition to the information of the system by which this recognition occurs.
The atomic information expose that just a couple of residues of these proteins determine the amino acids to which they bind and for that reason their particular functions. In addition, the study shows how the replacements of some residues for others in these positions in the different family members are responsible for customizing the uniqueness of recognition and transportation of some amino acids and not others.
The outcomes of this research study will now permit efforts to be directed towards compounds that could act on specific regions of these proteins, and to handle the disorders in which they participate, such as cancer and neurodegenerative diseases like Alzheimers illness.
Reference: “Structural basis for substrate specificity of heteromeric transporters of neutral amino acids” 29 November 2021, Proceedings of the National Academy of Sciences.DOI: 10.1073/ pnas.2113573118.
Led by IRB Barcelona and CNIO, the study has been performed in collaboration with the groups headed by Víctor Guallar, at the Barcelona Supercomputing Center (BSC) and Lucía Díaz, at the biotech Nostrum Biodiscovery.
The research study has been possible thanks to the financing from the “la Caixa” Foundation, which, through its CaixaResearch program, promotes the finest initiatives to deal with illness that cause the greatest effect worldwide, such as cardiovascular, neurological, contagious, and oncological conditions.
The study has also received financing from the Ministry of Science and Innovation, the Instituto de Salud Carlos III, the Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER), the European Regional Development Fund, and the Government of Catalonia.