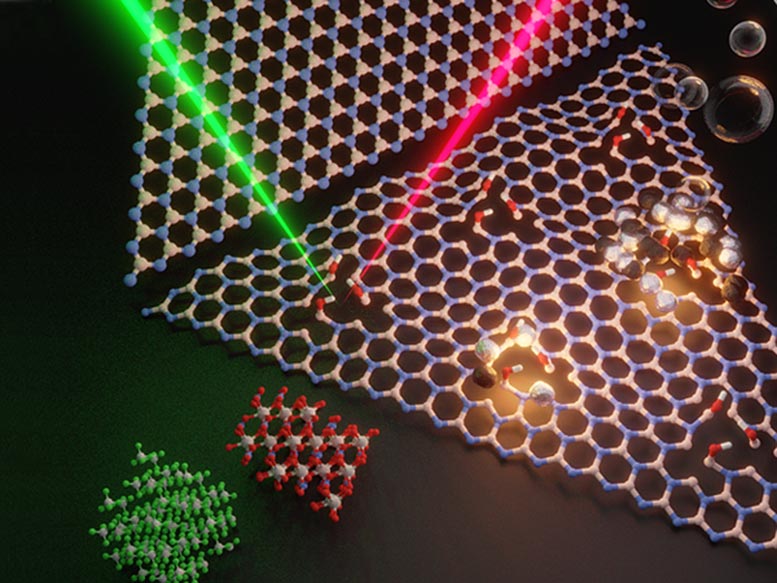
Scientists shine a light with a color or energy on the product and get a different color from the defect. In addition, the figure shows hydrogen bubbles being generated from these problems that include driver atoms (dark and gray spheres attached to the vacancies).” The catalytic converter in your gas cars and truck has the valuable metal platinum in it to process the conversion of damaging gases into less harmful gases,” said Jose Mendoza-Cortes, assistant professor of chemical engineering and products science at Michigan State University.” I think we are showing that product that is expected to be inert can be activated by developing and controlling defects on the product,” Terrones stated. If it works for boron nitride, it needs to work for any other material.”
If hBN was not inert and chemically active, that would allow more utilizes for it, consisting of being a helpful, affordable catalyst support comparable to graphene. This would be beneficial for practical applications like in a gasoline-powered auto or to transform carbon for helping in reducing greenhouse gases to other products.
” The catalytic converter in your gas automobile has the rare-earth element platinum in it to process the conversion of hazardous gases into less hazardous gases,” stated Jose Mendoza-Cortes, assistant teacher of chemical engineering and products science at Michigan State University. “However, this is costly due to the fact that you need to put in a lot of platinum atoms for the catalysis. Now envision that you just need to put a couple of, and still get the exact same performance.”
Platinum is likewise used as a driver for many other types of practical chemical responses, and the platinum atoms that carry out the conversion are normally on the surface, while the ones listed below are just there as structural support.
” In this study, we have used defective hBN as structural support, which is more affordable, while exposing the majority of the platinum atom for performing chemical responses,” Mendoza-Cortes stated.
The flaws in the hBN are the secret to the materials chemical activity. The researchers made flaws, tiny holes, in the materials by means of a process called cryomilling, which involves supercooling a material and then reducing it by means of cryogenic grinding.
The holes are so small that they can hold just one or 2 atoms of a precious metal at a time. By blending a metal salt, nanostructures as little as an atom or 2 onto the hBN substrate can be deposited, due to the reactivity of the hole-filled hBN.
” Since boron nitride doesnt respond with anything, then you can use this “holey” hBN as an assistance for catalysts if you lower a platinum, silver or gold salt into single atoms and position them in problems (holes) on the boron nitride surface,” stated Maurico Terrones, Verne M. Willaman Professor of physics and teacher of chemistry and products science at Penn State. “This is something completely brand-new, whichs what we showed here.”
Demonstrating this was considerable, as it was formerly believed that a product that is so inert might never ever end up being chemically active.
” The most tough part of this task was to convince the research study community that material that is as inert as hBN can be activated to have chemical reactivity, and work as the driver support,” Lei said. “During the procedure of evaluating our study, extra experiments that were suggested by the customers improved the work and assistance to convince the community.”
The experiments involved using high-end devices in the Materials Characterization Lab (MCL), part of the Materials Research Institute at Penn State. The computational and theoretical calculations were done at the Materials, Processes and Quantum Simulation Center (MUSiC) Lab and the Institute for Cyber-Enabled Research at Michigan State University.
” So, we needed to know what kind of defects we had in the material, and how can we show that we have the defects and its not something else?” Terrones said. “So, we did all these numerous really in-depth characterizations, including synchrotron radiation, to demonstrate that what we had remained in fact single-atom platinum, and not platinum clusters.”
Beyond experiments, the group also utilized modeling to show their concept.
” We showed and showed computationally and experimentally that we can make holes so small that they can hold only 1-, or 2-atoms of rare-earth elements at the time,” Mendoza-Cortes stated.
The capacity for applications for chemically active hBN is differed, consisting of more economical drivers, energy storage and sensing units. In addition, there is potential that their strategy could be utilized for activating other inert products or using other (valuable) metals.
” I think we are showing that product that is supposed to be inert can be activated by creating and controlling problems on the material,” Terrones stated. “We demonstrated that the necessary chemistry occurs at the atomic level. If it works for boron nitride, it needs to work for any other material.”
Referral: “Low temperature level activation of inert hexagonal boron nitride for metal deposition and single atom catalysis” by Yu Lei, Srimanta Pakhira, Kazunori Fujisawa, He Liu, Cynthia Guerrero-Bermea, Tianyi Zhang, Archi Dasgupta, Luis M.Martinez, Srinivasa Rao Singamaneni, Ke Wang, Jeff Shallenberger, Ana Laura Elías, Rodolfo Cruz-Silva, Morinobu Endo, Jose L. Mendoza-Cortes and Mauricio Terrones, 29 October 2021, Materials Today.DOI: 10.1016/ j.mattod.2021.09.017.
In addition to Lei, Mendoza-Cortes and Terrones, other authors of the research study include from the Indian Institute of Technology Indore, very first co-author Srimanta Pakhira, associate professor of physics. From Penn State, study authors consist of Kazunori Fujisawa, assistant research study teacher of physics; He Liu, doctoral research study assistant in chemistry at the time of the study; Tianyi Zhang, doctoral candidate in materials science and engineering at the time of the research study; Archi Dasgupta, graduate research study assistant in chemistry at the time of the study; Ke Wang, staff researcher with MCL; Jeff Shallenberger, associate director of the MCL; and Ana Laura Elías, research teacher of physics at the time of the research study. From the Yucatan Scientific Research Center, study authors consist of Cynthia Guerrero-Bermea, postdoctoral scientist. From the University of Texas at El Paso, study authors consist of Luis M. Martinez, college student in physics, and Srinivasa Rao Singamaneni, assistant teacher of physics. From Shinshu University (Japan), research study authors consist of Rodolfo Cruz-Silva, specially selected professor in the professors of engineering, and Morinobu Endo, Distinguished Professor Emeritus.
The research study was partially supported by the National Science Foundation. This work was supported in part through computational resources and services provided by the Institute for Cyber-Enabled Research at Michigan State University.
Defects in the lattice structure of hexagonal boron nitride can be detected with photoluminescence. Researchers shine a light with a color or energy on the product and get a different color from the problem. In addition, the figure shows hydrogen bubbles being produced from these flaws which contain catalyst atoms (dark and gray spheres connected to the jobs). Credit: Elizabeth Flores-Gomez Murray, Yu Lei and Kazunori Fujisawa
Showing that a material believed to be always chemically inert, hexagonal boron nitride (hBN), can be turned chemically active holds potential for a brand-new class of drivers with a vast array of applications, according to a global group of scientists.
hBN is a layered material and monolayers can be exfoliated like in graphene, another two-dimensional material. Nevertheless, there is a key distinction in between the two.
” While hBN shares comparable structure as graphene, the strong polar bonds between the boron and nitride atoms makes hBN unlike graphene because it is chemically inert and thermally stable at heat,” stated Yu Lei, postdoctoral scholar in physics at Penn State and first co-author in the research study released in Materials Today.