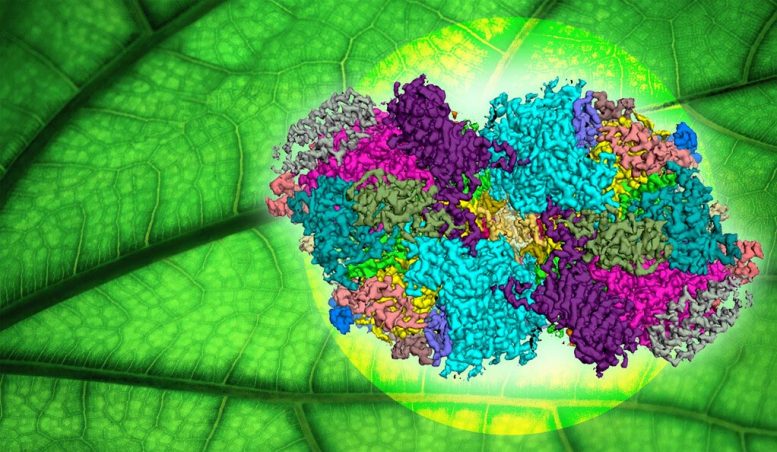
This illustration includes a cryo-EM “map” of the photosystem II complex. It is a 3D reconstruction, based on two-dimensional cryo-EM images, with different protein subunits of the complex colored individually. Credit: Yale University
A Yale-led group of chemists has actually unveiled the plans for a key enzyme that might consist of design concepts for a brand-new generation of synthetic solar fuel drivers.
The research study, led by Yales Gary Brudvig and Christopher Gisriel, uses cryo-electron microscopy on a microbe called Synechocystis to get an extreme close-up photo of Photosystem II, the enzyme in photosynthesis that uses water as a solar fuel, enabling scientists to observe how the enzyme works.
The research study, which appears in the journal Proceedings of the National Academy of Sciences, was co-authored by scientists from the University of California-Riverside, Boston College, and City University of New York.
Photosynthesis is the system by which plants and particular bacteria, like Synechocystis, use sunlight to manufacture food from carbon dioxide and water– and fill the environment with oxygen as a byproduct. At the heart of photosynthesis is Photosystem II, an enzyme that oxides water particles, removing their electrons to use as fuel.
Scientists have actually long looked for methods to mimic this procedure to develop more effective solar fuel catalysts, by studying Photosystem II from Synechocystis. But without a clear image of Photosystem IIs molecular structure in Synechocystis, it has actually been challenging for researchers to comprehend the results of their experiments.
Previous work led by Yale developed a picture of Photosytem II from Synechocystis in an “immature” phase, before the enzyme can water oxidation. That work permitted the researchers to better comprehend how the enzyme is developed.
In the new research study, the scientists had the ability to see the enzyme in Synechocystis in its mature, active type, with all of the protein subunits and activity that is present during water oxidation. The observation, enabled by cryo-electron microscopy technology at Yales West Campus, provides one of the closest, most detailed looks ever accomplished for Photosystem II in Synechocystis.
” At this resolution, we can see amino acids, small-molecule co-factors, and water molecules that are used in the system of water oxidation,” stated Brudvig, the Benjamin Silliman Professor of Chemistry in the Faculty of Arts and Sciences and director of the Energy Sciences Institute at Yales West Campus. Brudvig is the studys matching author.
” In some cases, we can even see the contribution of individual protons,” Brudvig included.
With this brand-new, up-close view of Photosystem II from Synechocystis, the researchers say theyll have the ability to present tiny modifications to the enzyme– such as mutating individual amino acids– to see how those changes affect the enzymes function.
” The main goal is to comprehend the chemistry of water oxidation,” stated Gisriel, a postdoctoral associate in chemistry and the research studys first author. “What weve done here provides a platform from which we can deconstruct the system, offering the style concepts for synthetic solar fuel drivers.”
Recommendation: “High-resolution cryo-electron microscopy structure of photosystem II from the mesophilic cyanobacterium, Synechocystis sp. PCC 6803 ″ by Christopher J. Gisriel, Jimin Wang, Jinchan Liu, David A. Flesher, Krystle M. Reiss, Hao-Li Huang, Ke R. Yang, William H. Armstrong, M. R. Gunner, Victor S. Batista, Richard J. Debus and Gary W. Brudvig, 22 December 2021, Proceedings of the National Academy of Sciences.DOI: 10.1073/ pnas.2116765118.
Co-authors of the research study from Yale are Jimin Wang, Jinchan Liu, David Flesher, Krystle Reiss, Hao-Li Huang, Ke Yang, and Victor Batista. Additional co-authors are William Armstrong from Boston College, M.R. Gunner from City College of New York, and Richard Debus of the University of California-Riverside.
The U.S. Department of Energys Office of Basic Energy Sciences and the National Institutes of Health moneyed the research study.