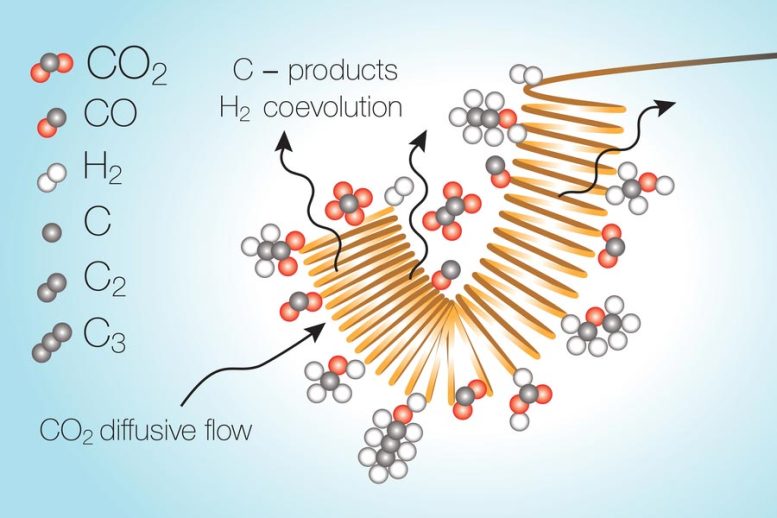
The perpetrator turns out to be a local deficiency of the carbon dioxide gas right next to the electrodes being used to catalyze the conversion. The thermal processes require extremely high temperature, and they do not produce extremely high-value chemical products, which is a difficulty with the light-activated processes as well, he states. The reactions take place as a stream of liquid electrolyte with the carbon dioxide dissolved in it passes over a metal catalytic surface that is electrically charged. As the carbon dioxide gets converted, it leaves behind an area in the electrolyte stream where it has actually essentially been utilized up, and so the reaction within this depleted zone turns towards water splitting instead. The concentration of carbon dioxide next to the catalyst dictates the products that are made.
MIT scientists have identified a problem that tends to limit chemical processes for turning co2 into fuel or other useful chemicals– and methods of dealing with that issue. Credit: Courtesy of the Varanasi Lab
Study reveals why some efforts to convert the greenhouse gas into fuel have stopped working, and uses possible options.
They might make a significant damage in greenhouse gas emissions if scientists could find a method to chemically transform carbon dioxide into fuels or other items. But numerous such procedures that have actually seemed appealing in the laboratory have not performed as anticipated in scaled-up formats that would appropriate for use with a power plant or other emissions sources.
Now, scientists at MIT have actually recognized, measured, and modeled a major reason for poor efficiency in such conversion systems. The offender turns out to be a regional deficiency of the co2 gas right beside the electrodes being utilized to catalyze the conversion. The problem can be eased, the team found, by merely pulsing the existing off and on at particular periods, enabling time for the gas to develop back up to the required levels next to the electrode.
The findings, which could stimulate progress on developing a variety of materials and designs for electrochemical co2 conversion systems, were published on January 11, 2022, in the journal Langmuir, in a paper by MIT postdoc Álvaro Moreno Soto, college student Jack Lake, and professor of mechanical engineering Kripa Varanasi.
” Carbon dioxide mitigation is, I believe, one of the essential challenges of our time,” Varanasi says. While much of the research study in the location has actually concentrated on carbon capture and sequestration, in which the gas is pumped into some kind of deep underground tank or converted to an inert solid such as limestone, another appealing opportunity has been converting the gas into other carbon substances such as methane or ethanol, to be utilized as fuel, or ethylene, which functions as a precursor to helpful polymers.
There are numerous ways to do such conversions, including electrochemical, thermocatalytic, photothermal, or photochemical procedures. “Each of these has problems or obstacles,” Varanasi says. The thermal processes need extremely high temperature level, and they dont produce extremely high-value chemical items, which is a challenge with the light-activated procedures as well, he says. “Efficiency is always at play, always an issue.”
The group has focused on the electrochemical methods, with an objective of getting “higher-C items”– compounds which contain more carbon atoms and tend to be higher-value fuels since of their energy per weight or volume. In these reactions, the greatest challenge has been suppressing competing responses that can occur at the very same time, especially the splitting of water molecules into oxygen and hydrogen.
The responses happen as a stream of liquid electrolyte with the co2 dissolved in it passes over a metal catalytic surface area that is electrically charged. However as the co2 gets transformed, it leaves a region in the electrolyte stream where it has actually essentially been used up, therefore the reaction within this diminished zone turns towards water splitting instead. This undesirable reaction utilizes up energy and considerably minimizes the overall efficiency of the conversion procedure, the scientists found.
” Theres a number of groups working on this, and a number of catalysts that are out there,” Varanasi states. “In all of these, I believe the hydrogen co-evolution becomes a bottleneck.”
One way of counteracting this depletion, they found, can be achieved by a pulsed system– a cycle of merely turning off the voltage, stopping the reaction and offering the co2 time to spread out back into the diminished zone and reach functional levels again, and after that resuming the response.
The concentration of carbon dioxide next to the driver determines the items that are made. “If you want to be able to make a system that works at industrial scale, you require to be able to run things over a long duration of time,” Varanasi states, “and you require to not have these kinds of effects that lower the efficiency or dependability of the process.”
The team studied 3 various catalyst products, including copper, and “we actually focused on making certain that we understood and can quantify the exhaustion effects,” Lake states. In the process they had the ability to develop a dependable and basic way of keeping track of the performance of the conversion process as it takes place, by determining the altering pH levels, a procedure of level of acidity, in the systems electrolyte.
In their tests, they used more sophisticated analytical tools to identify response products, including gas chromatography for analysis of the gaseous products, and nuclear magnetic resonance characterization for the systems liquid items. However their analysis showed that the basic pH measurement of the electrolyte beside the electrode during operation might provide an enough step of the performance of the reaction as it progressed.
This ability to easily keep track of the response in real-time might eventually cause a system optimized by machine-learning methods, controlling the production rate of the wanted compounds through continuous feedback, Moreno Soto says.
Now that the process is understood and measured, other approaches to reducing the co2 depletion may be developed, the scientists state, and might easily be evaluated using their approaches.
This work shows, Lake states, that “no matter what your catalyst product is” in such an electrocatalytic system, “youll be impacted by this problem.” And now, by using the model they established, its possible to identify precisely what kind of time window requires to be evaluated to get a precise sense of the products overall performance and what type of system operations could maximize its efficiency.
Recommendation: “Transient Effects Caused by Gas Depletion throughout Carbon Dioxide Electroreduction” by Álvaro Moreno Soto, Jack R. Lake and Kripa K. Varanasi, 11 January, Langmuir.DOI: 10.1021/ acs.langmuir.1 c02540.
The research was supported by Shell, through the MIT Energy Initiative.