Calico and tortoiseshell felines have various colored spots of fur since just one of their two X chromosomes is triggered in each cell.
How does this sex distinction in fur color occur biologically? As it turns out, cells with XX chromosomes experience X-inactivation: The X chromosome from one moms and dad is switched off in some cells, while the X chromosome acquired from the other parent is shut off in others. In the cells of female calico cats, X-inactivation can result in splotches of orange and black fur if one X chromosome comes from a moms and dad with orange fur and the other X chromosome comes from a moms and dad with black fur.
X-inactivation takes place since organisms like people and felines need only one X chromosome to operate properly. To make sure the right “dosage,” one of the X chromosomes is turned off in every cell.
Because X-inactivation occurs just in those individuals with more than one X chromosome, scientists like me have actually been taking a look at how the genes that escape inactivation on the 2nd X affect the health of individuals with XX chromosomes. Weve found that for particular conditions, cell sex may be at the heart of the matter.
A change of heart
One disease that X chromosome escape genes partially regulate is aortic valve stenosis, a condition in which the part of the heart that controls blood circulation to the rest of the body stiffens and narrows. Much like a person attempting to press open a door with rusty hinges, the heart gets worn out.
Hearts with aortic valve stenosis should pump harder to press blood through a narrowed aortic valve to the remainder of the body. Credit: SuneErichsen/Wikimedia Commons, CC BY-SA
People with XX chromosomes generally determine as female, and people with XY chromosomes usually recognize as male. When an orange feline mates with a black feline, female offspring, which normally acquire one X chromosome from each parent, will have a mixture of orange and black fur– one X chromosome encodes for orange fur while the other encodes for black fur. As it turns out, cells with XX chromosomes experience X-inactivation: The X chromosome from one moms and dad is turned off in some cells, while the X chromosome inherited from the other moms and dad is turned off in others. In the cells of female calico felines, X-inactivation can lead to splotches of orange and black fur if one X chromosome comes from a parent with orange fur and the other X chromosome comes from a parent with black fur.
Generally, people with XX chromosomes have increased scarring, called fibrosis, whereas individuals with XY chromosomes have increased calcium deposits.
How many X chromosomes you have can impact your health.
A lot of mammals, consisting of people, have two sex chromosomes, X and Y. People with XX chromosomes typically determine as female, and people with XY chromosomes usually determine as male.
Before I became a biomedical engineer studying how sex chromosomes affect the heart, I learnt more about one curious function of X chromosomes in my high school science class, with the calico feline example.
Female calico cats usually have orange and black splotches of fur, due to the fact that the gene that defines coat color is found on the X chromosome. When an orange cat mates with a black feline, female offspring, which usually acquire one X chromosome from each parent, will have a mixture of orange and black fur– one X chromosome encodes for orange fur while the other encodes for black fur. For this factor, male cats, which typically have one X and one Y chromosome, have solid orange or black coats.
My laboratory research studies how sex chromosomes can affect cardiovascular conditions like AVS. Generally, people with XX chromosomes have actually increased scarring, called fibrosis, whereas individuals with XY chromosomes have increased calcium deposits.
By and large, scientists think sex hormones drive sex differences in valve tissue stiffening. Decreasing estrogen levels throughout menopause can worsen heart fibrosis. Nevertheless, research studies on heart disease in XX and XY mice have actually discovered that sex distinctions still persist even after surgically excising the reproductive organs that produce sex hormonal agents.
My team and I assumed that the genes that leave X-inactivation, being unique to individuals with XX chromosomes, may be driving these differences in valve stiffening. To check this concept, we established bioengineered models of valve tissue using hydrogels. Hydrogels simulate the tightness of valve tissue much better than the standard petri meal medium, enabling us to study heart cells in an environment that more carefully looks like the body.
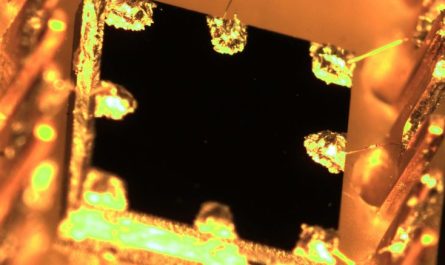
Heart tissue with XX chromosomes has a greater concentration of cells (colored green, with blue nuclei) that promote scarring than do cells with XY chromosomes. Credit: Brian Aguado, CC BY-NC-ND
We discovered that the cells we grew on our hydrogel models were able to replicate the sex differences seen in valve tissue– particularly, valve cells with XX chromosomes had more scarring than cells with XY chromosomes. Additionally, when we reduced the activity of genes that left X-inactivation, we were able to reduce scarring in XX chromosome cells.
Our next step was to use our models to figure out which treatments work best for AVS based upon cell sex. We found that XX valve cells were less delicate than XY cells to these drugs that targeted genes that promote scarring. Drugs that specifically target genes that leave X-inactivation, however, have a stronger result on XX cells.
Equitable look after all
Sex and gender variations in heart disease are rampant. For example, women are less likely than men to be recommended cardiovascular medications in spite of guideline suggestions, and transgender individuals have higher rates of cardiovascular disease than do cisgender folks.
Our work takes another step towards accomplishing equity in developing medical rehabs for cardiovascular disease. By taking sex chromosomes into consideration, my team and I believe that treatment strategies can be optimized for everybody, regardless of cell “seXX.”.
Written by Brian Aguado, Assistant Professor, University of California San Diego.
This post was very first published in The Conversation.