The brand-new strategy controls gene activity without changing the DNA series of the genome by targeting chemical modifications that help plan genes in our chromosomes and control their activity. Since these modifications happen not in, however on top of genes, they are called epigenetic, from the Greek epi “over” or “above” the genes. In their work, Levy and her coworkers focused on a complex of proteins called PRC2 that silences genes by attaching a small particle, called a methyl group, to a protein that packages genes called histones. Cas9 is the protein utilized in the gene modifying process called CRISPR. Existing thinking is that these promotor areas are close to the gene, within in 30 DNA base pairs, they found for this gene the promoter region was more than 500 base pairs away.
Hannele Ruohola Baker and Shiri Levy go over a brand-new strategy for awakening silenced genes in Ruohola-Bakers lab at the University of Washington School of Medicine Credit: Thatcher Heldring/UW Medicine Institute for Stem Cell and Regenerative Medicine.
Method allows scientists to toggle on individual genes that regulate cell development, function, and advancement.
By combining CRISPR technology with a protein developed with expert system, it is possible to awaken private dormant genes by disabling the chemical “off switches” that silence them. Scientists from the University of Washington School of Medicine in Seattle describe this finding in the journal Cell Reports.
The method will enable scientists to comprehend the role individual genes play in typical cell development and development, in aging, and in such diseases as cancer, stated Shiri Levy, a postdoctoral fellow in UW Institute for Stem Cell and Regenerative Medicine (ISCRM) and the lead author of the paper.
” The appeal of this approach is we can securely upregulate particular genes to affect cell activity without permanently changing the genome and trigger unintentional errors,” Levy stated.
The project was led by Hannele Ruohola-Baker, teacher of biochemistry and associate director of ISCRM. The AI-designed protein was developed at the UW Medicine Institute for Protein Design (IPD) under the management of David Baker, likewise a professor of biochemistry and head of the IPD.
The new strategy controls gene activity without altering the DNA series of the genome by targeting chemical adjustments that assist package genes in our chromosomes and control their activity. Due to the fact that these adjustments take place not in, however on top of genes, they are called epigenetic, from the Greek epi “over” or “above” the genes. The chemical modifications that manage gene activity are called epigenetic markers..
Researchers are especially thinking about epigenetic modifications due to the fact that not only do they affect gene activity in regular cell function, epigenetic markers build up with time, contribute to aging, and can impact of the health of future generations as we can pass them on to our children..
In their work, Levy and her associates focused on a complex of proteins called PRC2 that silences genes by attaching a little particle, called a methyl group, to a protein that packages genes called histones. These methyl groups should be refreshed so if PRC2 is blocked the genes it has silenced. it can be rekindled..
PRC2 is active throughout development however plays an especially important function throughout the very first days of life when embryonic cells distinguish into the various cell types that will form the tissues and organs of the growing embryo. PRC2 can be obstructed with chemicals, but they are inaccurate, impacting PRC2 function throughout the genome. The objective of the UW researchers was to find a way to block PRC2 so that only one gene at a time would be impacted.
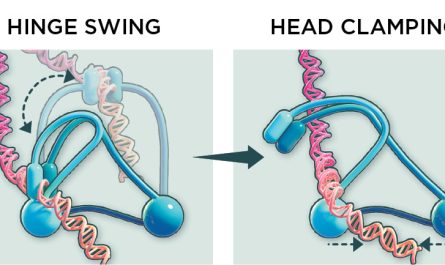
To do this, David Baker and his colleagues use AI to create a protein that would bind to PRC2 and obstruct a protein the PRC2 utilizes to modify the histones. Ruohola-Baker and Levy then fused this designed protein with a handicapped version of a protein called Cas9..
Cas9 is the protein used in the gene editing procedure called CRISPR. Cas9 uses and binds RNA as an address-tag. The system allows scientists, by synthesizing a specific “address-tag” RNA, to bring Cas9 to an accurate place in genome and therefore cut and splice genes at particular websites. In this experiment, however, the cutting function of the Cas9 protein is handicapped so the genomic DNA sequence is unchanged. As a result, its called dCas9, for “dead.” However, the Cas9 function as a “automobile” to deliver freight to a specific area stays active. The AI-designed stopping protein was the freight of the dCas9-RNA construct. “dCas9 is like UBER,” says Levy, “It will take you anywhere on the genome you desire to go. The guide RNA resembles a traveler, informing the UBER where to go.”.
In the new paper, Levy and her associates reveal that by utilizing this method, they were able to obstruct PRC2 and selectively turn on 4 various genes. They were also able to show they could transdifferentiate induced pluripotent stem cells to placental progenitor cells by merely switching on 2 genes..
” This technique permits us to avoid bombarding cells with various development elements and gene activators and repressors to get them to differentiate,” Levy stated. “Instead, we can target specific sites on the gene transcription promoters region, lift those marks and let the cell do the rest in a natural, holistic way. “.
The researchers were able to reveal how the strategy can be used to find the place of particular PRC2-controlled regulatory regions from where specific genes are activated. Present thinking is that these promotor regions are close to the gene, within in 30 DNA base sets, they discovered for this gene the promoter region was more than 500 base sets away.
” This was a very essential finding,” stated Ruohola-Baker. “TATA boxes are spread throughout the genome, and present thinking in biology is that the crucial TATA boxes are very near to the gene transcription site and the others dont seem to matter. The power of this tool is that it can find the important PRC2 reliant components, in this case TATA boxes that matter.”.
Epigenetic modifications decorate broad areas of the genome in normal and abnormal cells. The very little functional system for the epigenetic modification stays poorly understood, Ruohola-Baker notes, “With these 2 advances, AI-designed proteins and CRISPR innovation, we can now find the precise epigenetic marks that are important for gene expression, discover the guidelines and use them to control cell function, drive cell distinction and develop 21st century treatments.”.
Referral: “dCas9 combination to computer-designed PRC2 inhibitor reveals practical TATA box in distal promoter region” by Shiri Levy, Logeshwaran Somasundaram, Infencia Xavier Raj, Diego Ic-Mex, Ashish Phal, Sven Schmidt, Weng I. Ng, Daniel Mar, Justin Decarreau, Nicholas Moss, Ammar Alghadeer, Henrik Honkanen, Jay Sarthy, Nicholas Vitanza, R. David Hawkins, Julie Mathieu, Yuliang Wang, David Baker, Karol Bomsztyk and Hannele Ruohola-Baker, 1 March 2022, Cell Reports.DOI: 10.1016/ j.celrep.2022.110457.
This work was supported by grants from the National Institutes of Health (R01GM097372, R01GM97372-03S1, R01GM083867, 1P01GM081619, R42HG010855, U01CA246503), Department of Defense (PR203328 W81XWH-21-1-0006), American Heart Association (19IPLOI34760143) and the Washington Research Foundation, ISCRM Fellows program, and Brotman Baty Institute (BBI) for Precision Medicine.