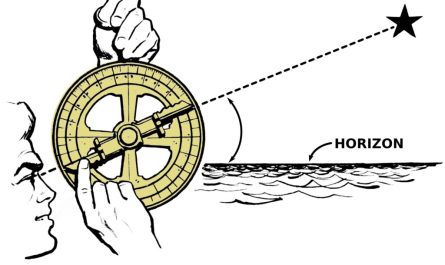
Illustration of the animals studied. Credit: Woranop Sukparangsi
An ancient fish called a “living fossil” has assisted scientists in getting a much deeper understanding of stem cells, which might potentially lead to developments in stem cell research study and the creation of synthetic organs.
A beating heart, an intricate organ responsible for pumping blood throughout the body. Not something typically associated with lab settings such as a Petri meal.
However, this understanding may change in the future as research study progresses toward the development of synthetic organs, which have the potential to conserve the lives of those with organ failure.
By University of Copenhagen – The Faculty of Health and Medical Sciences
January 22, 2023
Pluripotent stem cells are stem cells that can develop into all other cells. If we understand how the pluripotent stem cells establish into a heart, then we are one step better to replicating this process in a lab.
That way, you can begin to separate what is truly crucial for stem cells and use that to enhance how you grow stem cells in a meal,” says Ph.D. student Elena Morganti.
The researchers looked at the stem cell genes from over 40 animals. While the areas of these proteins understood to be important for stem cells do not change, species-specific distinctions in apparently unrelated regions of these proteins modify their orientation, possibly affecting how well it supports pluripotency.
To create artificial organs you first have to understand stem cells and the genetic guidelines that govern their impressive homes.
Professor Joshua Mark Brickman at the Novo Nordisk Foundation Center for Stem Cell Medicine (reNEW) has actually discovered the evolutionary origins of a master gene that acts on a network of genes instructing stem cells
” The first step in stem cell research study is to comprehend the gene regulatory network that supports so-called pluripotent stem cells. Comprehending how their function was refined in advancement can help supply understanding about how to construct much better stem cells,” states Joshua Mark Brickman.
Pluripotent stem cells are stem cells that can turn into all other cells. For instance, heart cells. We are one action more detailed to duplicating this process in a lab if we comprehend how the pluripotent stem cells develop into a heart.
A living fossil is the essential to comprehending stem cells.
The pluripotent property of stem cells– indicating that the cells can turn into any other cell– is something that has traditionally been connected with mammals.
Now Joshua Mark Brickman and his coworkers have found that the master gene that controls stem cells and supports pluripotency also exists in a fish called coelacanth. In mice and humans, this gene is called OCT4 and they discovered that the coelacanth version might change the mammalian one in mouse stem cells.
In addition to the fact that the coelacanth remains in a different class from mammals, it has actually also been called a living fossil, since around 400 million years ago it became the kind it has today. It has fins shaped like limbs and is for that reason thought to resemble the very first animals to move from the sea onto land.
” By studying its cells, you can go back in evolution, so to speak,” discusses Assistant Professor Molly Lowndes.
Assistant Professor Woranop Sukparangsi continues: “The central factor controlling the gene network in stem cells is found in the coelacanth. This shows that the network currently existed early in advancement, potentially as far back as 400 million years ago.”
And by studying the network in other species, such as this fish, the scientists can distill what the fundamental principles that support a stem cell are.
” The charm of returning in development is that the organisms end up being easier. They have only one copy of some important genes rather of many versions. That method, you can begin to separate what is truly essential for stem cells and use that to improve how you grow stem cells in a dish,” states Ph.D. student Elena Morganti.
Sharks, kangaroos, and mice
In addition to the researchers learning that the network around stem cells is much older than formerly thought, and found in ancient types, they also found out how precisely evolution has customized the network of genes to support pluripotent stem cells.
The researchers looked at the stem cell genes from over 40 animals. Sharks, kangaroos, and mice. The animals were picked to supply an excellent tasting of the main branch points in advancement.
The scientists used expert system to build three-dimensional models of the different OCT4 proteins. The scientists could see that the general structure of the protein is preserved throughout evolution. While the areas of these proteins known to be important for stem cells do not change, species-specific distinctions in apparently unrelated regions of these proteins modify their orientation, potentially affecting how well it supports pluripotency.
” This a very amazing finding about development that would not have actually been possible prior to the introduction of new innovations. You can see it as evolution skillfully believing, we do not tinker with the engine in the cars and truck, but we can move the engine around and enhance the drive train to see if it makes the car go quicker,” says Joshua Mark Brickman.
Recommendation: “Evolutionary origin of vertebrate OCT4/POU5 functions in supporting pluripotency” by Woranop Sukparangsi, Elena Morganti, Molly Lowndes, Hélène Mayeur, Melanie Weisser, Fella Hammachi, Hanna Peradziryi, Fabian Roske, Jurriaan Hölzenspies, Alessandra Livigni, Benoit Gilbert Godard, Fumiaki Sugahara, Shigeru Kuratani, Guillermo Montoya, Stephen R. Frankenberg, Sylvie Mazan and Joshua M. Brickman, 21 September 2022, Nature Communications.DOI: 10.1038/ s41467-022-32481-z.
The research study is a collaborative task spanning Australia, Japan, and Europe, with essential tactical collaborations with the groups of Sylvie Mazan at the Oceanological Observatory of Banyuls-sur-Mer in France and teacher Guillermo Montoya at Novo Nordisk Foundation Center for Protein Research at the University of Copenhagen.