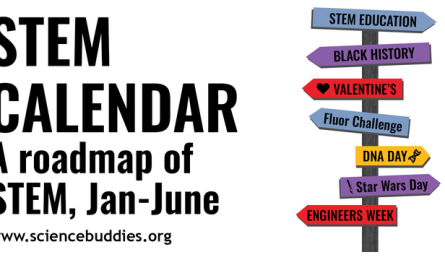
Photomicographs of an initially red single crystal demonstrate how it transitions to yellow during dehydration at -20 ° C. Credit: Alan Eaby, very first released in Nature, Vol. 616, 13 April 2022, by Springer Nature
A crystalline substance with nanoscale channels was found to launch water at temperature levels as low as -70 ° C, possibly revolutionizing materials designed for climatic water extraction and substantially minimizing energy expenses connected with the procedure.
Scientists at Stellenbosch University have found that a crystalline compound can release water at temperature levels as low as -70 ° C when subjected to nanoscale confinement, as reported in the journal Nature. The findings could have considerable ramifications for products designed to extract water from the atmosphere. The research group, led by Prof. Len Barbour, discovered that the narrow channels within the crystal structure, simply one nanometer wide, enable water to stay mobile at sub-zero temperatures, absorbing and releasing water far below its regular freezing point. This discovery opens a brand-new field of research and potential applications, consisting of the possibility of dramatically minimizing the energetic costs of atmospheric water harvesting.
Researchers have discovered yet another amazing element of the strange and fantastic behavior of water– this time when subjected to nanoscale confinement at sub-zero temperatures.
The research group, led by Prof. Len Barbour, found that the narrow channels within the crystal structure, simply one nanometer wide, permit water to remain mobile at sub-zero temperatures, releasing and absorbing water far below its regular freezing point. While we are familiar with materials designed to absorb water, it is extremely unusual for a material that soaks up water quickly to lose it similarly quickly”.
Theoretical modeling by Prof. Esterhuysen and MSc trainee Dirkie Myburgh revealed that water uptake causes slight modifications in the electronic residential or commercial properties of the crystals, causing them to turn red. I thought that there was something incorrect with the experimental setup or the temperature level controller, as crystal hydrates are not supposed to launch water at such low temperature levels,” he explains.
This glass transition ultimately causes the water to become trapped in the product at temperatures listed below -70 ° C.
Were it not for the color-changing behavior of the crystals in the first placeLocation they would not have become aware mindful the ultralow temperature water loss capability: “Who knows,” says Prof. Barbour, “there may might many lots of materials out there with the ability to release take in absorb launch at very low temperatures, such as metal-organic frameworks structures covalent organic frameworksStructures
The finding that a crystalline substance can easily quit water at temperatures as low as -70 ° C, published on April 12 in the journal Nature, has major implications for the advancement of materials created to extract water from the environment.
A team of supramolecular chemists at Stellenbosch University (SU), including Dr. Alan Eaby, Prof. Catharine Esterhuysen, and Prof. Len Barbour, made this discovery while attempting to comprehend the peculiar behavior of a type of crystal that initially stimulated their interest about ten years back.
” Scientists are presently skilled at developing products that can soak up water,” Prof. Barbour discusses. It is much harder to get those materials (we call them hydrates) to then release the water without having to supply energy in the form of heat. As all of us know, energy is pricey and hardly ever entirely green.
The chemical substance in question was originally manufactured by Prof. Marcin Kwit, a specialist in organic stereochemistry at Adam Mickiewicz University in Poland. It was then taken shape and brought to Prof. Barbours lab for further study by postdoctoral fellow Dr. Agnieszka Janiak. This was primarily because of Prof. Barbours interest in ring-shaped molecules and how they form channels when loaded together in crystals.
Dr. Janiak observed that the crystals were yellow on some days and red on others. It didnt take her long to determine that the crystals would only turn red on days with humidity levels higher than 55%. When humidity levels fell below this level, the crystals would return to being yellow.
” Not just was this habits rather unusual,” Prof. Barbour describes, “it was likewise happening really fast. It seems the crystals were taking in water as quick at high humidity as it was losing it again at low humidity. While we recognize with materials created to absorb water, it is extremely uncommon for a material that soaks up water easily to lose it similarly easily”.
Why do these crystals have such special properties? This question started a nearly ten-year examination, which at first concentrated on discussing the system behind the color change. Theoretical modeling by Prof. Esterhuysen and MSc trainee Dirkie Myburgh revealed that water uptake triggers slight modifications in the electronic properties of the crystals, triggering them to turn red. With such remarkable properties, Prof. Barbour was persuaded that the crystals would likewise have other fascinating homes.
When PhD student Alan Eaby began dabbling with the material, that is. At first, he had concentrated on room temperature studies for his MSc research study but would later on turn his attention to measuring residential or commercial properties at lower temperature levels when he embarked on his PhD three years ago. He needed to know how the crystals would behave when subjected to different temperature levels and humidity levels: “I was captivated by the color modification and wanted to explore what was happening at the atomic scale,” he discusses.
Having found out about establishing instruments and techniques from Prof. Barbour, he started using non-standard techniques to understand the mechanisms of water uptake and release in the product.
One day, he observed something unusual happening at temperatures below no degrees Celsius. “I discovered that the crystal still changed color at sub-zero temperatures. I believed that there was something incorrect with the experimental setup or the temperature level controller, as crystal hydrates are not supposed to release water at such low temperatures,” he explains.
After great deals of conversations and coffee breaks with Profs Barbour and Esterhuysen, and tweaking the speculative setup several times, they understood that Alans observations could be discussed by the narrowness of the channels in the material. The channels in the crystal are just one nanometre broad– one-thousandth the diameter of a human hair.
It was currently understood that, at the nanoscale, water can stay mobile within channels at temperature levels listed below 0 ° C. However, this research study showed for the very first time that such channels can likewise enable the uptake and release of water at temperature levels far listed below its normal freezing point.
These animations showed that water particles in the nanochannels move about easily until cooled to -70 ° C, whereupon they undergo a “reversible structuring occasion” to resemble a glassy state. This glass transition eventually triggers the water to become caught in the material at temperatures listed below -70 ° C.
Were it not for the color-changing behavior habits the crystals in the first placeLocation they would not have actually ended up being of the ultralow temperature level loss capabilityAbility “Who knows,” says Prof. Barbour, “there may might many numerous materials products there with the ability capability release soak up absorb water at very really temperaturesTemperature levels such as metal-organic frameworks and covalent organic naturalStructures
Now that we do know that such behavior is possible, it opens an entire brand-new field of research and possible applications. Researchers can use this new information to determine other materials with comparable residential or commercial properties, and also utilize the principles weve developed to tweak the low-temperature release of water.
Recommendation: “Dehydration of a crystal hydrate at subglacial temperatures” by Alan C. Eaby, Dirkie C. Myburgh, Akmal Kosimov, Marcin Kwit, Catharine Esterhuysen, Agnieszka M. Janiak and Leonard J. Barbour, 12 April 2023, Nature.DOI: 10.1038/ s41586-023-05749-7.