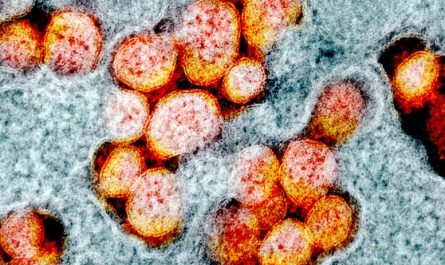
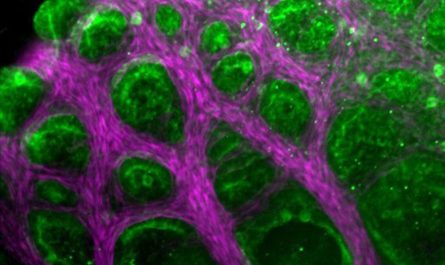
Todays catalysts for eliminating unburnt methane from natural gas engine exhaust are either ineffective at low, start-up temperatures or break down at higher operating temperatures. A brand-new single-atom driver established by SLAC National Accelerator Laboratory and Washington State University fixes both these issues and removes 90% of the methane.
Scientists have actually developed a catalyst utilizing single palladium atoms that can get rid of 90% of unburned methane from gas engine exhaust at low temperatures, providing the prospective to substantially lower methane emissions. Further research study is underway to advance this promising innovation toward commercialization.
Pioneering Catalyst Technology
A driver using a single or just a couple of palladium atoms removed 90% of unburned methane from natural gas engine exhaust at low temperatures in a recent research study. While more research requires to be done, the advance in single atom catalysis has the prospective to lower exhaust emissions of methane, among the worst greenhouse gases that traps heat at about 25 times the rate of co2.
Appealing Findings in Engine Emission Control
Reporting in the journal, Nature Catalysis, a research effort between Washington State University and SLAC National Accelerator Laboratory revealed that the single-atom driver was able to get rid of methane from engine exhaust at lower temperature levels, less than 350 degrees Celsius (662 degrees Fahrenheit), while maintaining response stability at greater temperatures.
Todays drivers for getting rid of unburnt methane from natural gas engine exhaust are either ineffective at low, start-up temperature levels or break down at greater operating temperature levels. A new single-atom driver established by SLAC National Accelerator Laboratory and Washington State University resolves both these problems and removes 90% of the methane. This illustration depicts private palladium atoms (white) removing methane (white bubbles) at the surface area of the catalyst.” Theres a huge drive towards utilizing natural gas, however when you use it for combustion engines, there will always be unburnt natural gas from the exhaust, and you have to find a method to get rid of that. “If you can eliminate 90% of the methane from the exhaust and keep the reaction stable, thats remarkable.”
” Its almost a self-modulating process which astonishingly conquers the challenges that people have actually been battling– low temperature inactivity and heat instability,” stated Yong Wang, Regents Professor in WSUs Gene and Linda Voiland School of Chemical Engineering and Bioengineering and a matching author on the paper.
The Challenges of Natural Gas Engines
Gas engines are used in about 30 million to 40 million lorries worldwide and are popular in Europe and Asia. The gas industry also uses them to run compressors that pump natural gas to peoples homes. They are normally thought about cleaner than gas or diesel engines, producing less carbon and particle pollution.
When these natural gas-powered engines start up, they produce unburnt, heat-trapping methane due to the fact that their catalytic converters dont work well at low temperatures. The drivers for methane removal are either inefficient at lower exhaust temperature levels or they significantly degrade at greater temperature levels.
The Importance of Methane Control
” Theres a huge drive towards utilizing gas, but when you use it for combustion engines, there will always be unburnt natural gas from the exhaust, and you have to discover a method to eliminate that. If not, you cause more severe global warming,” stated co-author Frank Abild-Pedersen, a staff scientist at SLAC National Accelerator Laboratory. “If you can eliminate 90% of the methane from the exhaust and keep the response steady, thats tremendous.”
A single-atom driver with the active metals singly dispersed on an assistance also uses every atom of the valuable and expensive metals, Wang added.
” If you can make them more reactive, thats the icing on the cake,” he said.
Reliable Methane Removal With Single Palladium Atoms
In their work, the scientists had the ability to reveal that their catalyst made from single palladium atoms on a cerium oxide support effectively removed methane from engine exhaust, even when the engine was simply beginning.
They discovered that trace quantities of carbon monoxide that are always present in engine exhaust played a crucial role in dynamically forming active websites for the response at space temperature. The carbon monoxide gas assisted the single atoms of palladium migrate to form 2- or three-atom clusters that effectively break apart the methane molecules at low temperature levels.
Then, as the exhaust temperatures increased, the sub-nanometer-sized clusters re-dispersed to single atoms once again so that the catalyst was thermally stable. This reversible procedure enables the driver to work effectively and uses every palladium atom the whole time the engine was running– including when it started cold.
Moving Towards Commercialization
Christopher Tassone, a SLAC National Accelerator Laboratory staff researcher and co-author on the paper, praised the groups capability to keep the palladium catalyst stable and extremely active, attributing it to the groups diverse expertise. He said, “We were actually able to discover a way to keep the supported palladium catalyst stable and highly active and since of the diverse competence throughout the group, to comprehend why this was occurring.”
While the researchers look for to further enhance the driver technology and much better understand why palladium acts in a different way from other valuable metals like platinum, they acknowledge that the research is some method from being incorporated into automobiles. Nevertheless, collaboration is underway with industry partners and Pacific Northwest National Laboratory to move the technology more detailed to commercialization.
Recommendation: “Dynamic and reversible transformations of subnanometre-sized palladium on ceria for effective methane elimination” by Dong Jiang, Gang Wan, Joakim Halldin Stenlid, Carlos E. García-Vargas, Jianghao Zhang, Chengjun Sun, Junrui Li, Frank Abild-Pedersen, Christopher J. Tassone and Yong Wang, 20 July 2023, Nature Catalysis.DOI: 10.1038/ s41929-023-00983-8.
In addition to Wang, Abild-Pedersen, and Tassone, Dong Jiang, senior research partner in the Voiland School, also led the work. The work was moneyed by the U.S. Department of Energys Office of Basic Energy Sciences.