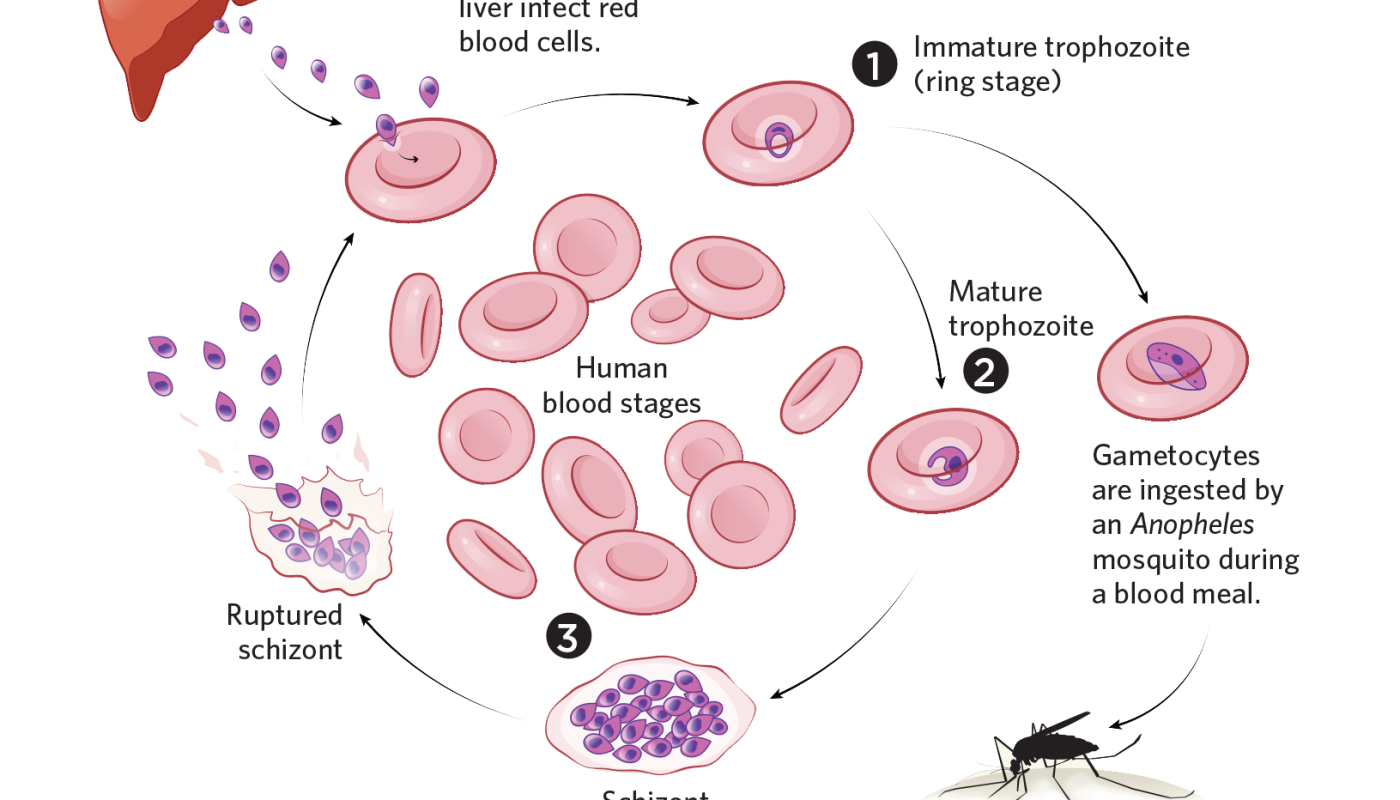
The plasmodium life cycle © TAMI TOLPAIn the early 2000s, RTS, S became the very first malaria vaccine to be evaluated in big clinical studies, led by GlaxoSmithKline and the Gates Foundation– funded PATH Malaria Vaccine Initiative. Participants were chosen at random to get three doses of the RTS, S vaccine or a control vaccine, each a month apart– with some receiving a fourth dosage 20 months after the. The success of future vaccines eventually boils down to financing, Tibenderana states; malaria vaccine research study is woefully underfunded.
The plasmodium life cycle © TAMI TOLPAIn the early 2000s, RTS, S became the first malaria vaccine to be checked in big scientific research studies, led by GlaxoSmithKline and the Gates Foundation– financed PATH Malaria Vaccine Initiative. Participants were chosen at random to get three dosages of the RTS, S vaccine or a control vaccine, each a month apart– with some getting a 4th dose 20 months after the. Still, the WHO determined that questions around security as well as practical elements of the vaccine rollout needed more research study, and the agency collaborated a carefully monitored pilot rollout of the vaccine. “Thats kind of frustrating after 30 years of vaccine advancement– that you ideally get the first certified vaccine in 2022, 2023, and there isnt enough of it by an aspect of ten,” Hill says. The success of future vaccines eventually boils down to funding, Tibenderana states; malaria vaccine research is woefully underfunded.