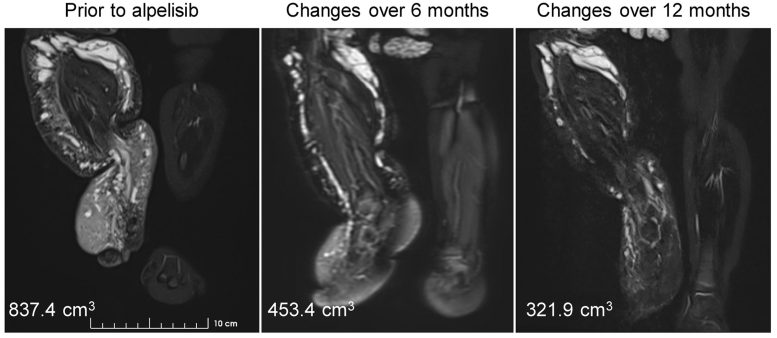
MRI shows the decrease in vascular malformations and leg size caused by alpelisib treatment. Credit: © 2022 Morin et al. Originally released in Journal of Experimental Medicine. https://doi.org/10.1084/jem.20212148
PIK3CA-related overgrowth spectrum (PROS) is a group of unusual, incurable disorders brought on by mutations in the PIK3CA gene that lead to the malformation and overgrowth of different parts of the body. A new report to be released today (January 26, 2022) in the Journal of Experimental Medicine ( JEM) describes the effective treatment of two young babies with PROS utilizing the breast cancer drug alpelisib.
PROS includes a group of diseases that, taken together, is believed to impact around 1 in 70,000 people. Mutations in the PIK3CA gene change the growth and expansion of cells, causing the malformation or extreme growth of different tissues, consisting of the brain, blood vessels, muscle, and bone, which often leads to serious impairment and sudden death.
Though some PROS signs can be eased through surgical treatment and other forms of palliative care, there are currently no medical treatments approved to treat the disease. However, alpelisib, a PIK3CA inhibitor just recently approved to deal with specific types of breast cancer, has actually shown appealing outcomes in both animal models of PROS and a little number of adult and older pediatric patients. The drug is now being examined in a series of larger medical trials, however, previously, there have actually been no data on alpelisibs effectiveness in young infants with PROS.
In the new JEM study, a group of scientists led by Professor Guillaume Canaud (Necker-Enfants Malades Hospital, AP-HP; Université Paris Descartes; Inserm) identified two young clients– a 9-month-old young boy and an 8-month-old woman– with a range of severe signs brought on by anomalies in the PIK3CA gene. These symptoms included extreme blood vessel malformations and unbalanced overgrowth of limbs and digits, as well as, in the kids case, an enlarged right brain hemisphere related to epileptic spasms.
Daily oral dosages of 25 mg alpelisib induced a considerable and quick medical improvement in all of these symptoms. In the ladys case, for instance, 12 months of alpelisib treatment reduced the quantity of vascular malformations and substantially decreased the size of her right leg, enabling her to stroll and stand with support, while the kids epileptic convulsions were significantly reduced.
The childrens total development appeared to be considerably enhanced, and neither client revealed any adverse results of alpelisib treatment. More analyses exposed that, at an everyday dose of 25 mg, the levels of alpelisib that accumulated in the childrens blood were much lower than the levels that can be safely tolerated by adults.
” Given the well-established safety profile of alpelisib at the approved 300-mg dose in adults, these low direct exposures support the continuous treatment of 25 mg alpelisib in these young clients with PROS,” Canaud says. “The results of alpelisib treatment in these two infants are encouraging, but they ought to be analyzed with care and will have to be validated by future research studies.”
Recommendation: “Treatment of 2 infants with PIK3CA-related overgrowth spectrum by alpelisib” 26 January 2022, Journal of Experimental Medicine.DOI: 10.1084/ jem.20212148.
MRI reveals the decrease in vascular malformations and leg size induced by alpelisib treatment. Alpelisib, a PIK3CA inhibitor recently approved to treat specific forms of breast cancer, has revealed promising outcomes in both animal designs of PROS and a little number of adult and older pediatric clients. The drug is now being examined in a series of larger scientific trials, but, until now, there have actually been no data on alpelisibs effectiveness in young babies with PROS.